 Found 237 hits with Last Name = 'li' and Initial = 'yz'
Found 237 hits with Last Name = 'li' and Initial = 'yz' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555
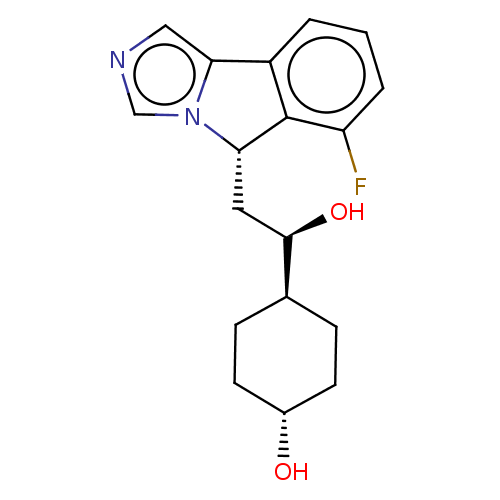
((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50139531

(CHEMBL165545 | Octyl-carbamic acid 3-octylcarbamoy...)Show InChI InChI=1S/C24H40N2O4/c1-3-5-7-9-11-13-18-25-23(27)29-21-16-15-17-22(20-21)30-24(28)26-19-14-12-10-8-6-4-2/h15-17,20H,3-14,18-19H2,1-2H3,(H,25,27)(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against phospholipase A2 of Naja mocambique mocambique |
Bioorg Med Chem Lett 14: 751-5 (2004)
BindingDB Entry DOI: 10.7270/Q2BK1BRZ |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50139529
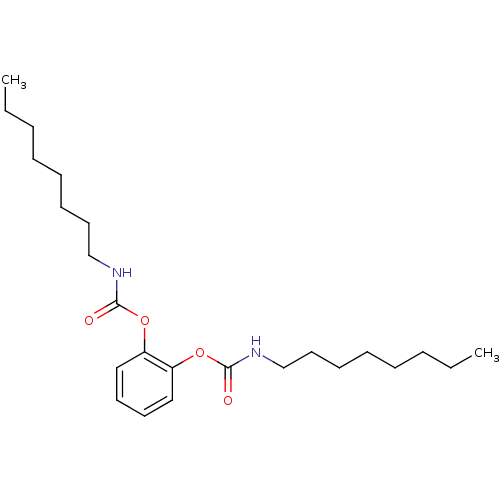
(CHEMBL166217 | Octyl-carbamic acid 2-octylcarbamoy...)Show InChI InChI=1S/C24H40N2O4/c1-3-5-7-9-11-15-19-25-23(27)29-21-17-13-14-18-22(21)30-24(28)26-20-16-12-10-8-6-4-2/h13-14,17-18H,3-12,15-16,19-20H2,1-2H3,(H,25,27)(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against phospholipase A2 of Naja mocambique mocambique |
Bioorg Med Chem Lett 14: 751-5 (2004)
BindingDB Entry DOI: 10.7270/Q2BK1BRZ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50139530

(CHEMBL434865 | Octyl-carbamic acid 4-octylcarbamoy...)Show InChI InChI=1S/C24H40N2O4/c1-3-5-7-9-11-13-19-25-23(27)29-21-15-17-22(18-16-21)30-24(28)26-20-14-12-10-8-6-4-2/h15-18H,3-14,19-20H2,1-2H3,(H,25,27)(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against phospholipase A2 of Naja mocambique mocambique |
Bioorg Med Chem Lett 14: 751-5 (2004)
BindingDB Entry DOI: 10.7270/Q2BK1BRZ |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50207089
(D-1-Methyltryptophan | D-1MT | Indoximod)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278725
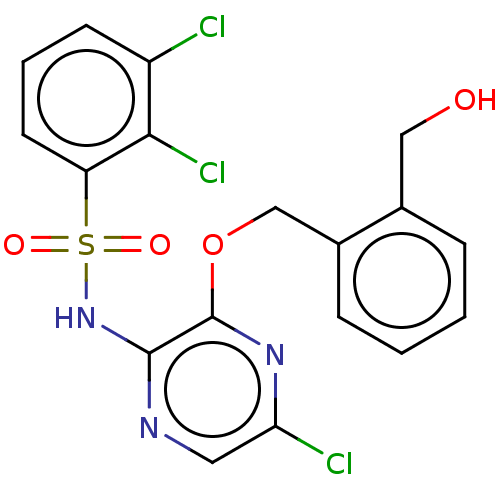
(CHEMBL4172769)Show SMILES OCc1ccccc1COc1nc(Cl)cnc1NS(=O)(=O)c1cccc(Cl)c1Cl Show InChI InChI=1S/C18H14Cl3N3O4S/c19-13-6-3-7-14(16(13)21)29(26,27)24-17-18(23-15(20)8-22-17)28-10-12-5-2-1-4-11(12)9-25/h1-8,25H,9-10H2,(H,22,24) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278758
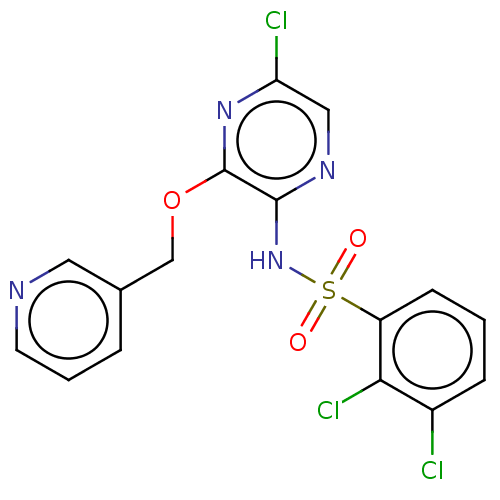
(CHEMBL4167445)Show SMILES Clc1cnc(NS(=O)(=O)c2cccc(Cl)c2Cl)c(OCc2cccnc2)n1 Show InChI InChI=1S/C16H11Cl3N4O3S/c17-11-4-1-5-12(14(11)19)27(24,25)23-15-16(22-13(18)8-21-15)26-9-10-3-2-6-20-7-10/h1-8H,9H2,(H,21,23) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Mus musculus) | BDBM50278798
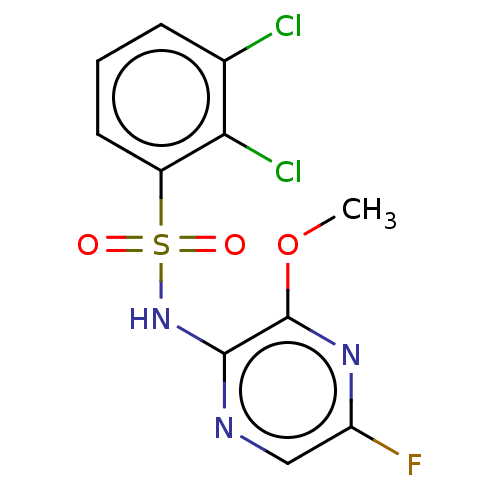
(CHEMBL4172635)Show InChI InChI=1S/C11H8Cl2FN3O3S/c1-20-11-10(15-5-8(14)16-11)17-21(18,19)7-4-2-3-6(12)9(7)13/h2-5H,1H3,(H,15,17) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278799
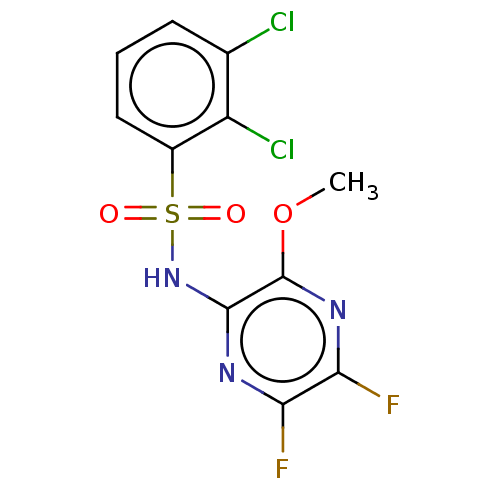
(CHEMBL4162364)Show InChI InChI=1S/C11H7Cl2F2N3O3S/c1-21-11-10(16-8(14)9(15)17-11)18-22(19,20)6-4-2-3-5(12)7(6)13/h2-4H,1H3,(H,16,18) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416
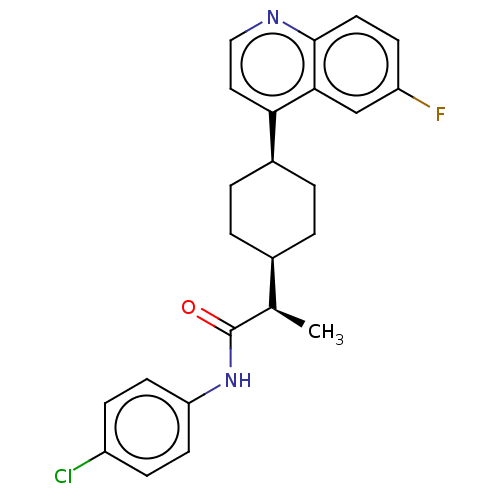
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278793
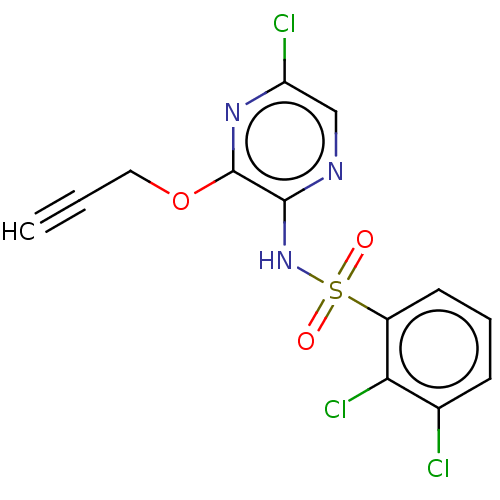
(CHEMBL4160218)Show SMILES Clc1cnc(NS(=O)(=O)c2cccc(Cl)c2Cl)c(OCC#C)n1 Show InChI InChI=1S/C13H8Cl3N3O3S/c1-2-6-22-13-12(17-7-10(15)18-13)19-23(20,21)9-5-3-4-8(14)11(9)16/h1,3-5,7H,6H2,(H,17,19) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278798

(CHEMBL4172635)Show InChI InChI=1S/C11H8Cl2FN3O3S/c1-20-11-10(15-5-8(14)16-11)17-21(18,19)7-4-2-3-6(12)9(7)13/h2-5H,1H3,(H,15,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Canis lupus familiaris) | BDBM50278798

(CHEMBL4172635)Show InChI InChI=1S/C11H8Cl2FN3O3S/c1-20-11-10(15-5-8(14)16-11)17-21(18,19)7-4-2-3-6(12)9(7)13/h2-5H,1H3,(H,15,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278727

(CHEMBL4173046)Show InChI InChI=1S/C11H8Cl2FN3O3S/c1-20-11-10(16-8(14)5-15-11)17-21(18,19)7-4-2-3-6(12)9(7)13/h2-5H,1H3,(H,16,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278794
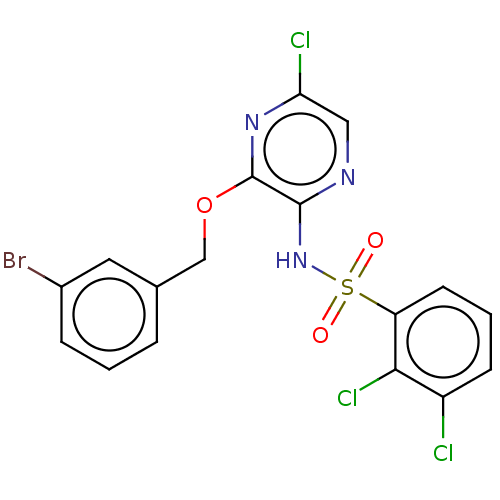
(CHEMBL4175323)Show SMILES Clc1cnc(NS(=O)(=O)c2cccc(Cl)c2Cl)c(OCc2cccc(Br)c2)n1 Show InChI InChI=1S/C17H11BrCl3N3O3S/c18-11-4-1-3-10(7-11)9-27-17-16(22-8-14(20)23-17)24-28(25,26)13-6-2-5-12(19)15(13)21/h1-8H,9H2,(H,22,24) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278798

(CHEMBL4172635)Show InChI InChI=1S/C11H8Cl2FN3O3S/c1-20-11-10(15-5-8(14)16-11)17-21(18,19)7-4-2-3-6(12)9(7)13/h2-5H,1H3,(H,15,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278797

(CHEMBL4160846)Show InChI InChI=1S/C12H10Cl3N3O4S/c1-22-12-11(17-10(15)7(5-19)16-12)18-23(20,21)8-4-2-3-6(13)9(8)14/h2-4,19H,5H2,1H3,(H,17,18) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 was determined |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Mus musculus) | BDBM50378979

(CHEMBL2011441)Show InChI InChI=1S/C9H7BrClN3O3S2/c1-17-9-8(12-4-5(10)13-9)14-19(15,16)7-3-2-6(11)18-7/h2-4H,1H3,(H,12,14) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CCR4 |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50562497
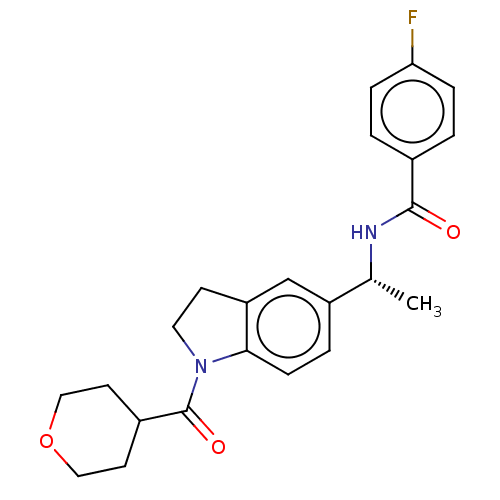
(CHEMBL4778760)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCc2c1)C(=O)C1CCOCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278801

(CHEMBL4165356)Show InChI InChI=1S/C11H8Cl3N3O3S/c1-20-11-10(16-8(13)5-15-11)17-21(18,19)7-4-2-3-6(12)9(7)14/h2-5H,1H3,(H,16,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278787

(CHEMBL4173861)Show InChI InChI=1S/C9H6Cl3N3O3S2/c1-18-8-7(13-2-5(11)14-8)15-20(16,17)9-6(12)4(10)3-19-9/h2-3H,1H3,(H,13,15) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50590774

(CHEMBL5203175) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278785
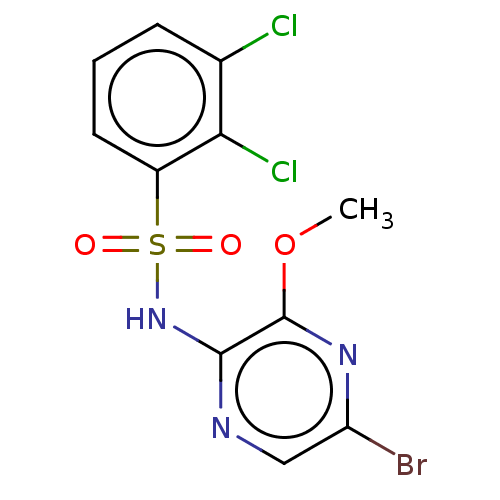
(CHEMBL4170431)Show InChI InChI=1S/C11H8BrCl2N3O3S/c1-20-11-10(15-5-8(12)16-11)17-21(18,19)7-4-2-3-6(13)9(7)14/h2-5H,1H3,(H,15,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Mus musculus) | BDBM50278724
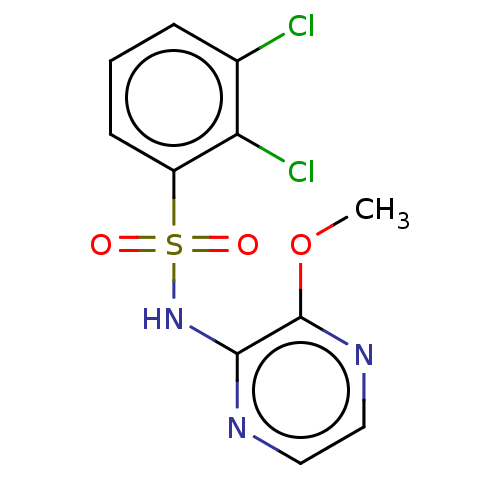
(CHEMBL4169247)Show InChI InChI=1S/C11H9Cl2N3O3S/c1-19-11-10(14-5-6-15-11)16-20(17,18)8-4-2-3-7(12)9(8)13/h2-6H,1H3,(H,14,16) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278723

(CHEMBL4162097)Show SMILES Clc1cnc(NS(=O)(=O)c2cccc(Cl)c2Cl)c(OCc2cncnc2)n1 Show InChI InChI=1S/C15H10Cl3N5O3S/c16-10-2-1-3-11(13(10)18)27(24,25)23-14-15(22-12(17)6-21-14)26-7-9-4-19-8-20-5-9/h1-6,8H,7H2,(H,21,23) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278800
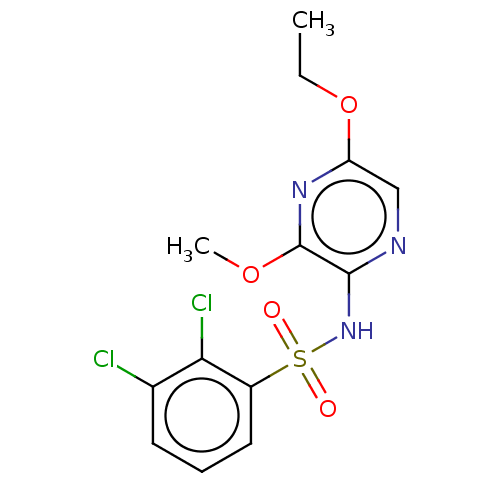
(CHEMBL4175972)Show InChI InChI=1S/C13H13Cl2N3O4S/c1-3-22-10-7-16-12(13(17-10)21-2)18-23(19,20)9-6-4-5-8(14)11(9)15/h4-7H,3H2,1-2H3,(H,16,18) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278786
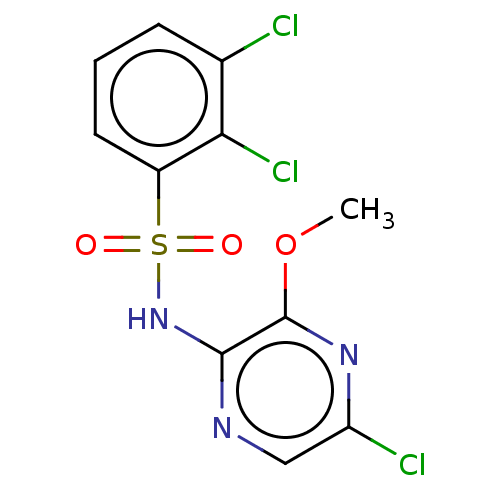
(CHEMBL4165939)Show InChI InChI=1S/C11H8Cl3N3O3S/c1-20-11-10(15-5-8(13)16-11)17-21(18,19)7-4-2-3-6(12)9(7)14/h2-5H,1H3,(H,15,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061174
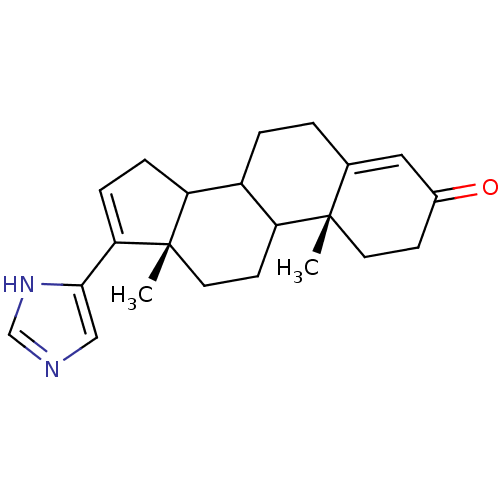
((10R,13S)-17-(1H-Imidazol-4-yl)-10,13-dimethyl-1,2...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cnc[nH]1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h6,11-13,16-18H,3-5,7-10H2,1-2H3,(H,23,24)/t16?,17?,18?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278749
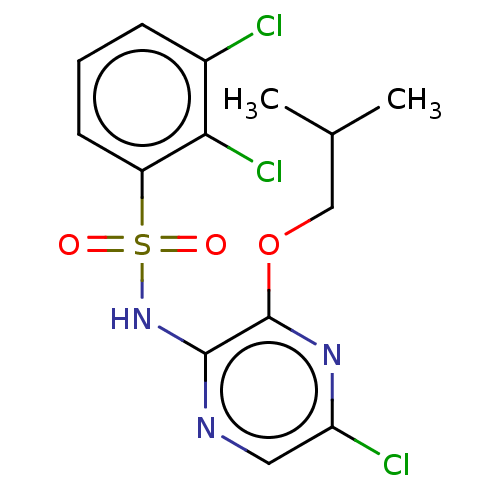
(CHEMBL4170833)Show SMILES CC(C)COc1nc(Cl)cnc1NS(=O)(=O)c1cccc(Cl)c1Cl Show InChI InChI=1S/C14H14Cl3N3O3S/c1-8(2)7-23-14-13(18-6-11(16)19-14)20-24(21,22)10-5-3-4-9(15)12(10)17/h3-6,8H,7H2,1-2H3,(H,18,20) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278724

(CHEMBL4169247)Show InChI InChI=1S/C11H9Cl2N3O3S/c1-19-11-10(14-5-6-15-11)16-20(17,18)8-4-2-3-7(12)9(8)13/h2-6H,1H3,(H,14,16) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278738

(CHEMBL4162555)Show InChI InChI=1S/C11H8Cl2FN3O3S/c1-20-11-10(15-5-8(12)16-11)17-21(18,19)7-4-2-3-6(14)9(7)13/h2-5H,1H3,(H,15,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278802

(CHEMBL4164738)Show InChI InChI=1S/C9H7Cl2N3O3S2/c1-17-8-7(12-2-3-13-8)14-19(15,16)9-6(11)5(10)4-18-9/h2-4H,1H3,(H,12,14) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278761
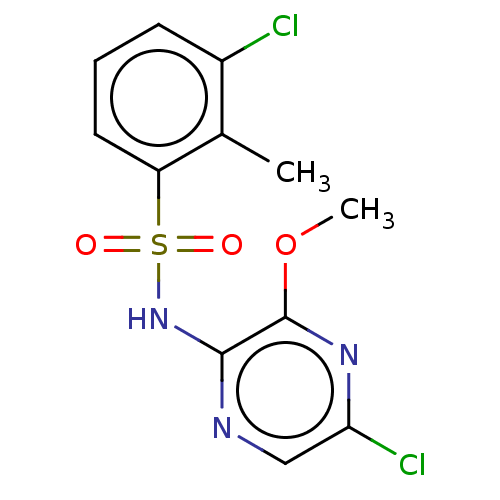
(CHEMBL4168237)Show InChI InChI=1S/C12H11Cl2N3O3S/c1-7-8(13)4-3-5-9(7)21(18,19)17-11-12(20-2)16-10(14)6-15-11/h3-6H,1-2H3,(H,15,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408273

(CHEMBL2112522)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ncc[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)13-14(21)3-4-16-17-5-6-19(20-23-11-12-24-20)22(17,2)10-8-18(16)21/h3,6,11-12,15-18,25H,4-5,7-10,13H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408267

(CHEMBL2112526)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1cnc[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h3,6,12-13,15-18,25H,4-5,7-11H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278759

(CHEMBL4168775)Show InChI InChI=1S/C12H11Cl2N3O4S/c1-21-12-11(15-5-7(6-18)16-12)17-22(19,20)9-4-2-3-8(13)10(9)14/h2-5,18H,6H2,1H3,(H,15,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Canis lupus familiaris) | BDBM50278724

(CHEMBL4169247)Show InChI InChI=1S/C11H9Cl2N3O3S/c1-19-11-10(14-5-6-15-11)16-20(17,18)8-4-2-3-7(12)9(8)13/h2-6H,1H3,(H,14,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408271

(CHEMBL2112517)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccn[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-10-7-15(25)13-14(21)3-4-16-17-5-6-19(20-9-12-23-24-20)22(17,2)11-8-18(16)21/h3,6,9,12,15-18,25H,4-5,7-8,10-11,13H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50590774

(CHEMBL5203175) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278724

(CHEMBL4169247)Show InChI InChI=1S/C11H9Cl2N3O3S/c1-19-11-10(14-5-6-15-11)16-20(17,18)8-4-2-3-7(12)9(8)13/h2-6H,1H3,(H,14,16) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in CHO cells coexpressing CD4+ assessed as inhibition of CCL22 induced Ca2+ mobilization |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061172
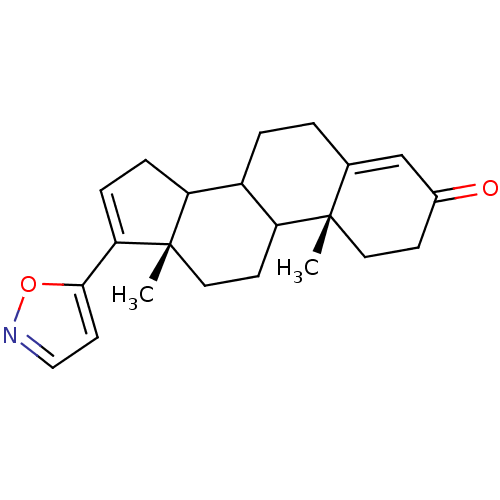
((10R,13S)-17-Isoxazol-5-yl-10,13-dimethyl-1,2,6,7,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1ccno1 |c:21,t:8| Show InChI InChI=1S/C22H27NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-23-25-20)22(17,2)11-8-18(16)21/h6,9,12-13,16-18H,3-5,7-8,10-11H2,1-2H3/t16?,17?,18?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061172

((10R,13S)-17-Isoxazol-5-yl-10,13-dimethyl-1,2,6,7,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1ccno1 |c:21,t:8| Show InChI InChI=1S/C22H27NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-23-25-20)22(17,2)11-8-18(16)21/h6,9,12-13,16-18H,3-5,7-8,10-11H2,1-2H3/t16?,17?,18?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278750
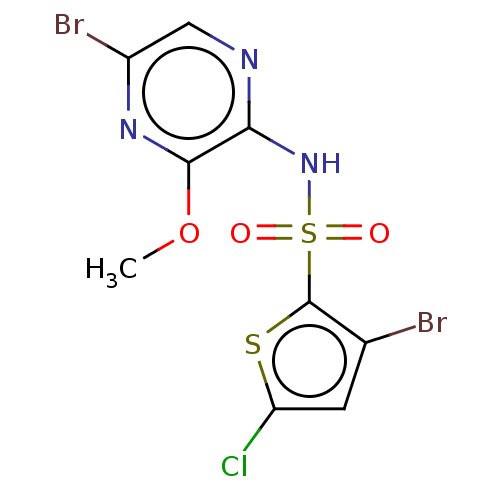
(CHEMBL4160139)Show InChI InChI=1S/C9H6Br2ClN3O3S2/c1-18-8-7(13-3-5(11)14-8)15-20(16,17)9-4(10)2-6(12)19-9/h2-3H,1H3,(H,13,15) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50278726
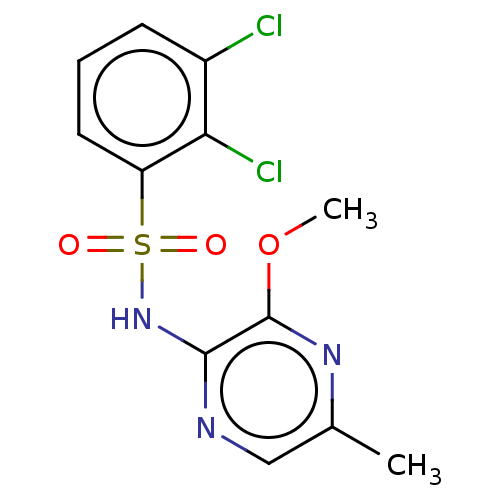
(CHEMBL4172568)Show InChI InChI=1S/C12H11Cl2N3O3S/c1-7-6-15-11(12(16-7)20-2)17-21(18,19)9-5-3-4-8(13)10(9)14/h3-6H,1-2H3,(H,15,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) |
ACS Med Chem Lett 8: 981-986 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00315
BindingDB Entry DOI: 10.7270/Q2KD21DK |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606613

(CHEMBL5219865)Show SMILES O[C@@H]1CCCC[C@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data