

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50061156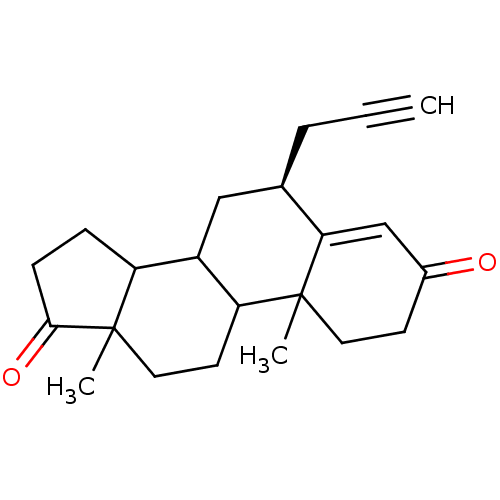 ((S)-10,13-Dimethyl-6-prop-2-ynyl-1,6,7,8,9,10,11,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 by nonlinear regression analysis | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50421840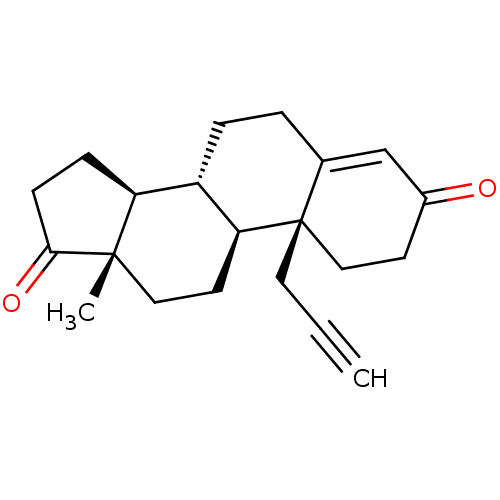 (MDL-18962 | PLOMESTANE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 by nonlinear regression analysis | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50405353 (CHEMBL346234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its competitive inhibitory activity against Cytochrome P450 19A1 with the use of human placental microsomal preparation | J Med Chem 29: 582-4 (1986) BindingDB Entry DOI: 10.7270/Q2VM4DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50031436 (10,13-Dimethyl-4-phenylsulfanyl-1,6,7,8,9,10,11,12...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its competitive inhibitory activity against Cytochrome P450 19A1 with the use of human placental microsomal preparation | J Med Chem 29: 582-4 (1986) BindingDB Entry DOI: 10.7270/Q2VM4DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50061153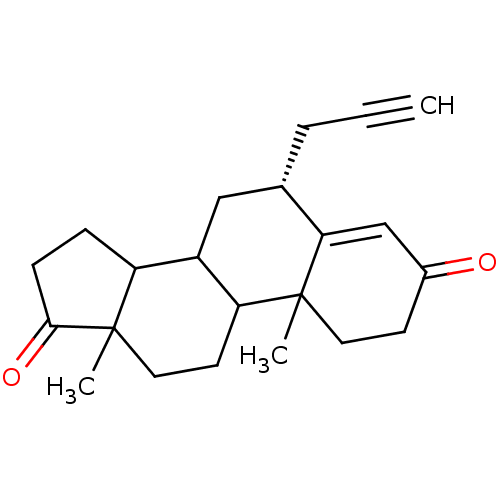 ((R)-10,13-Dimethyl-6-prop-2-ynyl-1,6,7,8,9,10,11,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 by nonlinear regression analysis | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50405354 (CHEMBL154104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its competitive inhibitory activity against Cytochrome P450 19A1 with the use of human placental microsomal preparation | J Med Chem 29: 582-4 (1986) BindingDB Entry DOI: 10.7270/Q2VM4DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50061154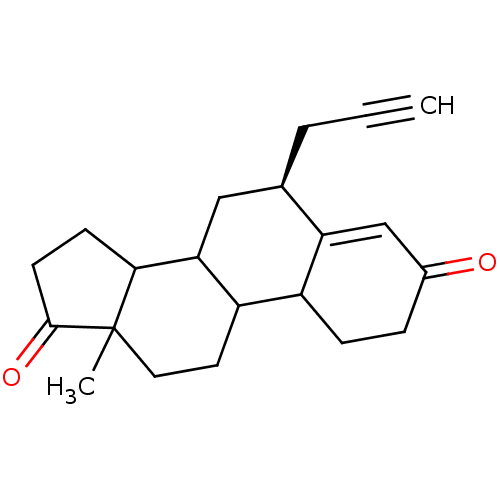 ((S)-13-Methyl-6-prop-2-ynyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 by nonlinear regression analysis | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50240798 ((8R,9S,10R,13S,14S)-4-Hydroxy-10,13-dimethyl-1,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its competitive inhibitory activity against Cytochrome P450 19A1 with the use of human placental microsomal preparation | J Med Chem 29: 582-4 (1986) BindingDB Entry DOI: 10.7270/Q2VM4DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50005871 (10,13-Dimethyl-4-methylsulfanyl-1,6,7,8,9,10,11,12...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its competitive inhibitory activity against Cytochrome P450 19A1 with the use of human placental microsomal preparation | J Med Chem 29: 582-4 (1986) BindingDB Entry DOI: 10.7270/Q2VM4DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50405356 (CHEMBL154918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its competitive inhibitory activity against Cytochrome P450 19A1 with the use of human placental microsomal preparation | J Med Chem 29: 582-4 (1986) BindingDB Entry DOI: 10.7270/Q2VM4DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50061155 ((R)-13-Methyl-6-prop-2-ynyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity for human placental microsome Cytochrome P450 19A1 by nonlinear regression analysis | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50405355 (CHEMBL152237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its competitive inhibitory activity against Cytochrome P450 19A1 with the use of human placental microsomal preparation | J Med Chem 29: 582-4 (1986) BindingDB Entry DOI: 10.7270/Q2VM4DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50061156 ((S)-10,13-Dimethyl-6-prop-2-ynyl-1,6,7,8,9,10,11,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human placental microsome Cytochrome P450 19A1 | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50421840 (MDL-18962 | PLOMESTANE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human placental microsome Cytochrome P450 19A1 | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50061153 ((R)-10,13-Dimethyl-6-prop-2-ynyl-1,6,7,8,9,10,11,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human placental microsome Cytochrome P450 19A1 | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50061155 ((R)-13-Methyl-6-prop-2-ynyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human placental microsome Cytochrome P450 19A1 | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50031436 (10,13-Dimethyl-4-phenylsulfanyl-1,6,7,8,9,10,11,12...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 | J Med Chem 38: 4135-8 (1995) BindingDB Entry DOI: 10.7270/Q2WS8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50061154 ((S)-13-Methyl-6-prop-2-ynyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human placental microsome Cytochrome P450 19A1 | J Med Chem 40: 3263-70 (1997) Article DOI: 10.1021/jm970325z BindingDB Entry DOI: 10.7270/Q23J3C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50031435 (10,13-Dimethyl-4-phenoxy-1,6,7,8,9,10,11,12,13,14,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 | J Med Chem 38: 4135-8 (1995) BindingDB Entry DOI: 10.7270/Q2WS8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50031437 ((R)-10,13-Dimethyl-7-phenylsulfanyl-1,6,7,8,9,10,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 | J Med Chem 38: 4135-8 (1995) BindingDB Entry DOI: 10.7270/Q2WS8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50031438 ((R)-10,13-Dimethyl-4-phenoxy-7-phenylsulfanyl-1,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 | J Med Chem 38: 4135-8 (1995) BindingDB Entry DOI: 10.7270/Q2WS8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||