

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using BTCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman ass... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50526787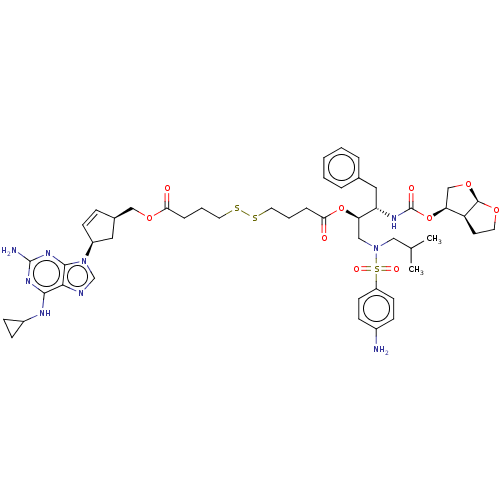 (CHEMBL4437966) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of P-gp in human CMEC/D3 cells using calcein-AM as substrate after 30 mins by flow cytometric analysis | J Med Chem 63: 2131-2138 (2020) Article DOI: 10.1021/acs.jmedchem.9b00779 BindingDB Entry DOI: 10.7270/Q28P640X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50526787 (CHEMBL4437966) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of P-gp in human CMEC/D3 cells using NBD-Aba as substrate after 30 mins by flow cytometric analysis | J Med Chem 63: 2131-2138 (2020) Article DOI: 10.1021/acs.jmedchem.9b00779 BindingDB Entry DOI: 10.7270/Q28P640X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535937 (CHEMBL4532806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535922 (CHEMBL4559797) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535923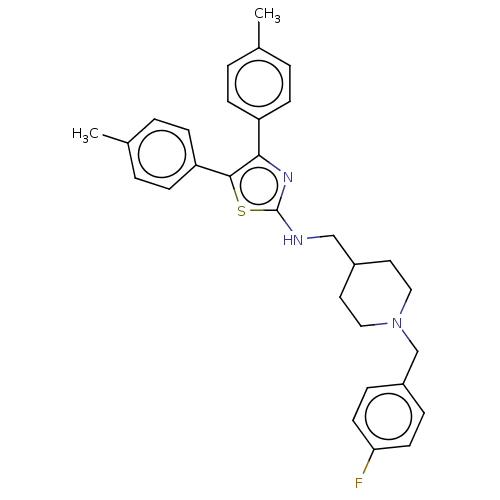 (CHEMBL4549453) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535950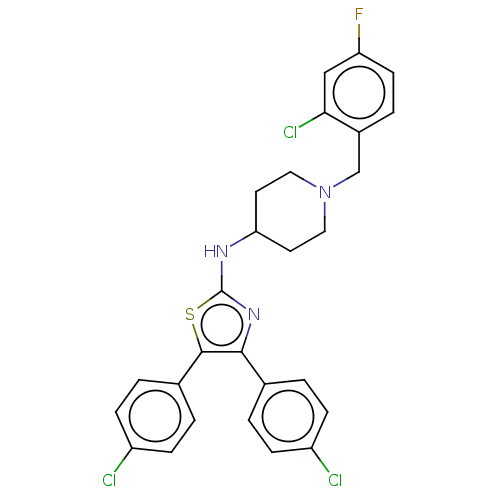 (CHEMBL4516508) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535938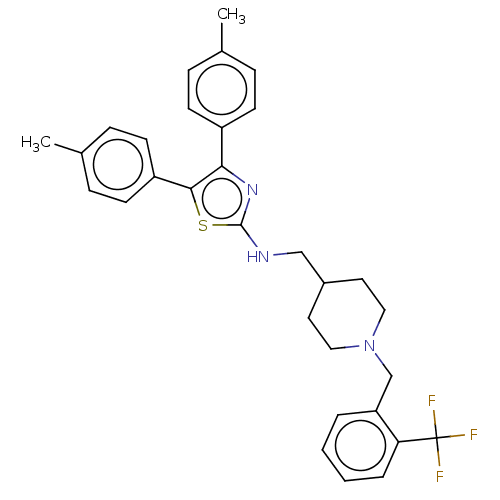 (CHEMBL4532372) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535952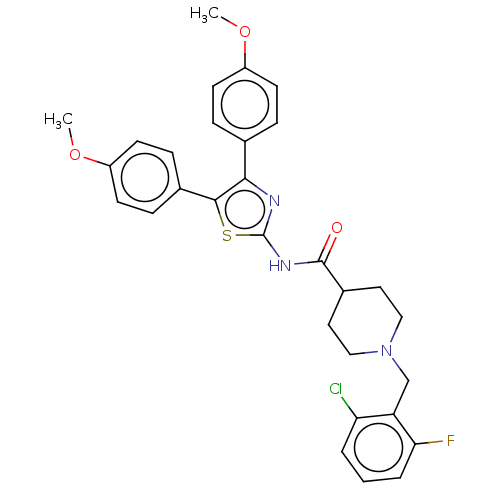 (CHEMBL4570156) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50526786 (CHEMBL4591618) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of P-gp in human CMEC/D3 cells using NBD-Aba as substrate after 30 mins by flow cytometric analysis | J Med Chem 63: 2131-2138 (2020) Article DOI: 10.1021/acs.jmedchem.9b00779 BindingDB Entry DOI: 10.7270/Q28P640X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535939 (CHEMBL4560673) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50526787 (CHEMBL4437966) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of P-gp in human 12D7-MDR cells using calcein-AM as substrate after 30 mins by flow cytometric analysis | J Med Chem 63: 2131-2138 (2020) Article DOI: 10.1021/acs.jmedchem.9b00779 BindingDB Entry DOI: 10.7270/Q28P640X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50526787 (CHEMBL4437966) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of P-gp in human 12D7-MDR cells using NBD-Aba as substrate after 30 mins by flow cytometric analysis | J Med Chem 63: 2131-2138 (2020) Article DOI: 10.1021/acs.jmedchem.9b00779 BindingDB Entry DOI: 10.7270/Q28P640X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535936 (CHEMBL4554666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535959 (CHEMBL4571742) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535958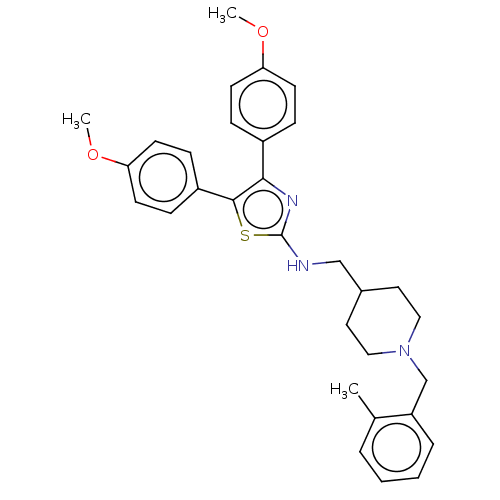 (CHEMBL4566786) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50526786 (CHEMBL4591618) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of P-gp in human CMEC/D3 cells using calcein-AM as substrate after 30 mins by flow cytometric analysis | J Med Chem 63: 2131-2138 (2020) Article DOI: 10.1021/acs.jmedchem.9b00779 BindingDB Entry DOI: 10.7270/Q28P640X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535951 (CHEMBL4529936) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535954 (CHEMBL4532149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535944 (CHEMBL4591964) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50526786 (CHEMBL4591618) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of P-gp in human 12D7-MDR cells using NBD-Aba as substrate after 30 mins by flow cytometric analysis | J Med Chem 63: 2131-2138 (2020) Article DOI: 10.1021/acs.jmedchem.9b00779 BindingDB Entry DOI: 10.7270/Q28P640X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535924 (CHEMBL4527101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535964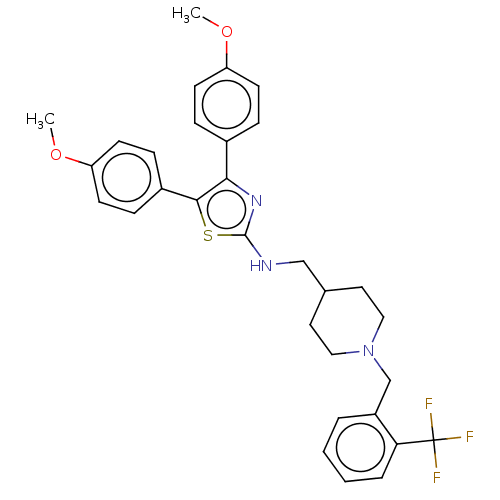 (CHEMBL4527770) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50535944 (CHEMBL4591964) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using BTCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman ass... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50535936 (CHEMBL4554666) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using BTCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman ass... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535963 (CHEMBL4544950) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50526786 (CHEMBL4591618) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of P-gp in human 12D7-MDR cells using calcein-AM as substrate after 30 mins by flow cytometric analysis | J Med Chem 63: 2131-2138 (2020) Article DOI: 10.1021/acs.jmedchem.9b00779 BindingDB Entry DOI: 10.7270/Q28P640X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535921 (CHEMBL4562938) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50535951 (CHEMBL4529936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using BTCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman ass... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535933 (CHEMBL4555730) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535956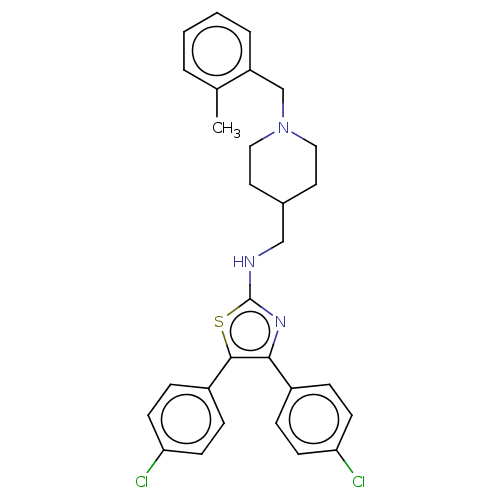 (CHEMBL4530559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535932 (CHEMBL4593917) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50535946 (CHEMBL4566613) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using BTCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman ass... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535961 (CHEMBL4540644) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535946 (CHEMBL4566613) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535931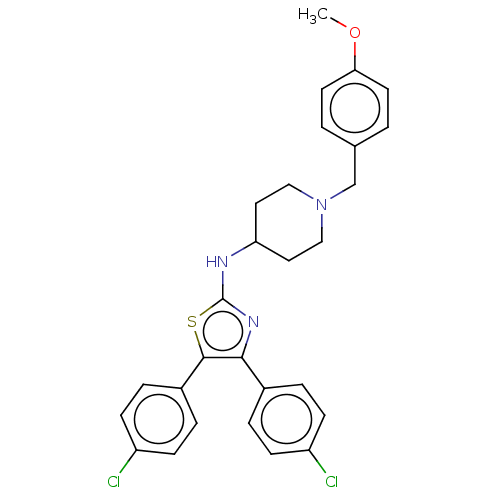 (CHEMBL4551316) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535941 (CHEMBL4520251) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM81939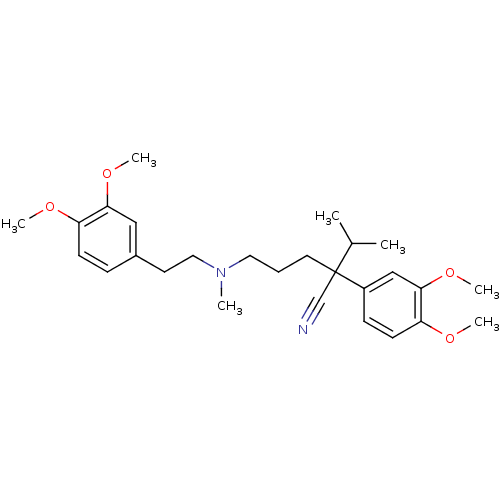 (CAS_52-53-9 | NSC_62969 | VERAPAMIL) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of P-gp in human 12D7-MDR cells using calcein-AM as substrate | J Med Chem 63: 2131-2138 (2020) Article DOI: 10.1021/acs.jmedchem.9b00779 BindingDB Entry DOI: 10.7270/Q28P640X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535926 (CHEMBL4549599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535935 (CHEMBL4533530) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using BTCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman ass... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535955 (CHEMBL4535748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535934 (CHEMBL4519072) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535953 (CHEMBL4593583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535943 (CHEMBL4548499) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50535925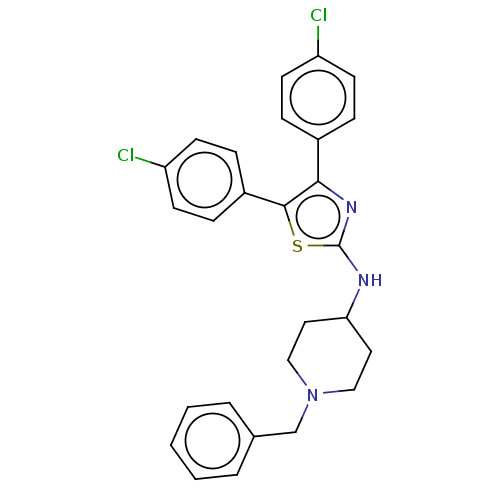 (CHEMBL4575730) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50535956 (CHEMBL4530559) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using BTCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman ass... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50535929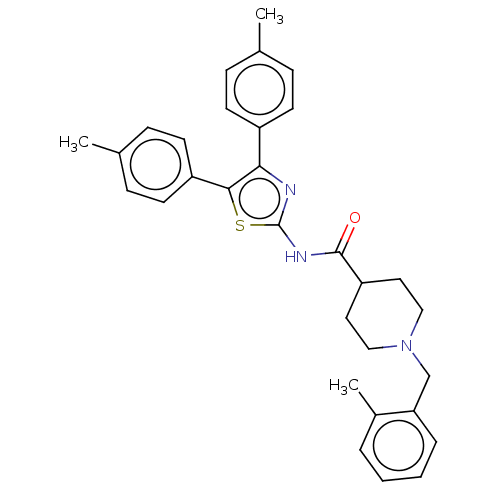 (CHEMBL4570655) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Maharaja Sayajirao University of Baroda Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using BTCI as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by Ellman ass... | J Med Chem 59: 5823-46 (2016) Article DOI: 10.1021/acs.jmedchem.6b00426 BindingDB Entry DOI: 10.7270/Q2SJ1Q44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |