 Found 301 hits with Last Name = 'ahmed' and Initial = 'e'
Found 301 hits with Last Name = 'ahmed' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50005480

((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minia University
Curated by ChEMBL
| Assay Description
Inhibition of [3H] colchicine binding to bovine brain tubulin incubated for 30 mins by scintillation proximity assay |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111697
BindingDB Entry DOI: 10.7270/Q2PG1W17 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50504214
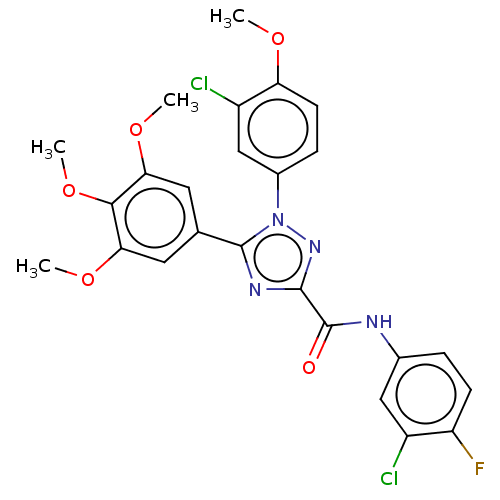
(CHEMBL4530425)Show SMILES COc1ccc(cc1Cl)-n1nc(nc1-c1cc(OC)c(OC)c(OC)c1)C(=O)Nc1ccc(F)c(Cl)c1 Show InChI InChI=1S/C25H21Cl2FN4O5/c1-34-19-8-6-15(12-17(19)27)32-24(13-9-20(35-2)22(37-4)21(10-13)36-3)30-23(31-32)25(33)29-14-5-7-18(28)16(26)11-14/h5-12H,1-4H3,(H,29,33) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minia University
Curated by ChEMBL
| Assay Description
Inhibition of [3H] colchicine binding to bovine brain tubulin incubated for 30 mins by scintillation proximity assay |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111697
BindingDB Entry DOI: 10.7270/Q2PG1W17 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50504215
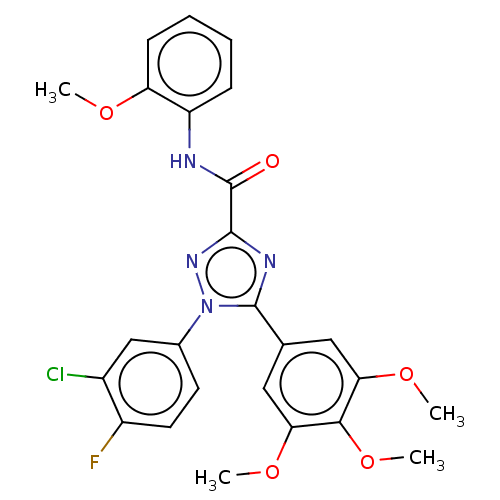
(CHEMBL4463939)Show SMILES COc1ccccc1NC(=O)c1nc(-c2cc(OC)c(OC)c(OC)c2)n(n1)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C25H22ClFN4O5/c1-33-19-8-6-5-7-18(19)28-25(32)23-29-24(31(30-23)15-9-10-17(27)16(26)13-15)14-11-20(34-2)22(36-4)21(12-14)35-3/h5-13H,1-4H3,(H,28,32) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minia University
Curated by ChEMBL
| Assay Description
Inhibition of [3H] colchicine binding to bovine brain tubulin incubated for 30 mins by scintillation proximity assay |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111697
BindingDB Entry DOI: 10.7270/Q2PG1W17 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50176468

(CHEMBL202223 | haloxysterol B)Show SMILES CC[C@H](C[C@@H](O)[C@@H](C)[C@H]1CCC2C3C=CC4=C[C@@H](O)C[C@H](O)[C@]4(C)C3CC[C@]12C)C(C)C |c:13,t:15| Show InChI InChI=1S/C29H48O3/c1-7-19(17(2)3)14-26(31)18(4)23-10-11-24-22-9-8-20-15-21(30)16-27(32)29(20,6)25(22)12-13-28(23,24)5/h8-9,15,17-19,21-27,30-32H,7,10-14,16H2,1-6H3/t18-,19+,21+,22?,23+,24?,25?,26+,27-,28+,29-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50176465
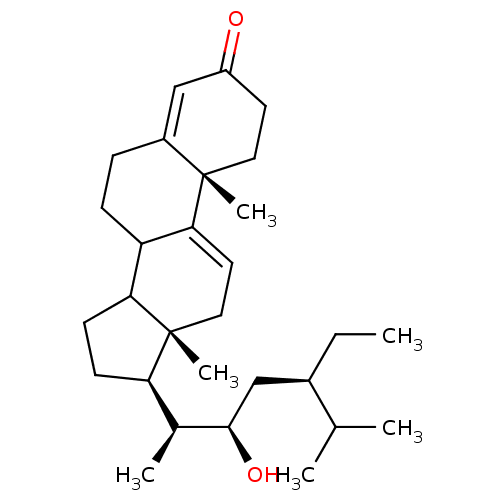
(CHEMBL382961 | haloxysterol C)Show SMILES CC[C@H](C[C@@H](O)[C@@H](C)[C@H]1CCC2C3CCC4=CC(=O)CC[C@]4(C)C3=CC[C@]12C)C(C)C |c:25,t:15| Show InChI InChI=1S/C29H46O2/c1-7-20(18(2)3)16-27(31)19(4)24-10-11-25-23-9-8-21-17-22(30)12-14-28(21,5)26(23)13-15-29(24,25)6/h13,17-20,23-25,27,31H,7-12,14-16H2,1-6H3/t19-,20+,23?,24+,25?,27+,28-,29+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50176467

(CHEMBL201253 | haloxysterol D)Show SMILES CC[C@H](C[C@@H](O)[C@@H](C)[C@H]1CCC2C3C[C@@H](O)[C@@]4(O)C[C@@H](O)C[C@H](O)[C@]4(C)C3CC[C@]12C)C(C)C Show InChI InChI=1S/C29H52O5/c1-7-18(16(2)3)12-24(31)17(4)21-8-9-22-20-14-26(33)29(34)15-19(30)13-25(32)28(29,6)23(20)10-11-27(21,22)5/h16-26,30-34H,7-15H2,1-6H3/t17-,18+,19-,20?,21+,22?,23?,24+,25-,26+,27+,28-,29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50176468

(CHEMBL202223 | haloxysterol B)Show SMILES CC[C@H](C[C@@H](O)[C@@H](C)[C@H]1CCC2C3C=CC4=C[C@@H](O)C[C@H](O)[C@]4(C)C3CC[C@]12C)C(C)C |c:13,t:15| Show InChI InChI=1S/C29H48O3/c1-7-19(17(2)3)14-26(31)18(4)23-10-11-24-22-9-8-20-15-21(30)16-27(32)29(20,6)25(22)12-13-28(23,24)5/h8-9,15,17-19,21-27,30-32H,7,10-14,16H2,1-6H3/t18-,19+,21+,22?,23+,24?,25?,26+,27-,28+,29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50176470

(24-ethyl-cholest-7-ene-3,5,6-triol | CHEMBL201951)Show SMILES CCC(CC[C@@H](C)[C@H]1CC[C@H]2C3=CC(O)C4(O)C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(C)C |t:11| Show InChI InChI=1S/C29H50O3/c1-7-20(18(2)3)9-8-19(4)23-10-11-24-22-16-26(31)29(32)17-21(30)12-15-28(29,6)25(22)13-14-27(23,24)5/h16,18-21,23-26,30-32H,7-15,17H2,1-6H3/t19-,20?,21+,23-,24+,25+,26?,27-,28-,29?/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50176472
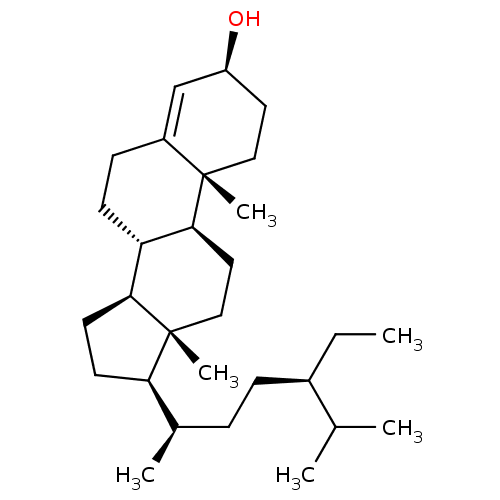
(CHEMBL202496 | lawsaritol)Show SMILES CC[C@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CCC4=C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(C)C |t:14| Show InChI InChI=1S/C29H50O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h18-21,23-27,30H,7-17H2,1-6H3/t20-,21-,23+,24+,25-,26+,27+,28+,29-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50176466

(24-ethylcholest-6-ene-3,5-diol | CHEMBL201866)Show SMILES CCC(CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3C=CC4(O)C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(C)C |c:12| Show InChI InChI=1S/C29H50O2/c1-7-21(19(2)3)9-8-20(4)24-10-11-25-23-13-17-29(31)18-22(30)12-16-28(29,6)26(23)14-15-27(24,25)5/h13,17,19-26,30-31H,7-12,14-16,18H2,1-6H3/t20-,21?,22+,23+,24-,25+,26+,27-,28-,29?/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50176466

(24-ethylcholest-6-ene-3,5-diol | CHEMBL201866)Show SMILES CCC(CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3C=CC4(O)C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(C)C |c:12| Show InChI InChI=1S/C29H50O2/c1-7-21(19(2)3)9-8-20(4)24-10-11-25-23-13-17-29(31)18-22(30)12-16-28(29,6)26(23)14-15-27(24,25)5/h13,17,19-26,30-31H,7-12,14-16,18H2,1-6H3/t20-,21?,22+,23+,24-,25+,26+,27-,28-,29?/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50176469

(CHEMBL202221 | haloxysterol A)Show SMILES CC[C@H](C[C@@H](O)[C@@H](C)[C@H]1CCC2C3CC=C4C[C@@H](O)C[C@H](O)[C@]4(C)C3CC[C@]12C)C(C)C |t:14| Show InChI InChI=1S/C29H50O3/c1-7-19(17(2)3)14-26(31)18(4)23-10-11-24-22-9-8-20-15-21(30)16-27(32)29(20,6)25(22)12-13-28(23,24)5/h8,17-19,21-27,30-32H,7,9-16H2,1-6H3/t18-,19+,21+,22?,23+,24?,25?,26+,27-,28+,29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50176473

((24S)-ethylcholesta-7,9(11),22(E)-triene-3b-ol | C...)Show SMILES CC[C@H](\C=C\[C@@H](C)[C@H]1CC[C@H]2[C@@H]3C=CC4C[C@@H](O)CC[C@]4(C)C3=CC[C@]12C)C(C)C |c:12,24| Show InChI InChI=1S/C29H46O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h8-11,15,19-26,30H,7,12-14,16-18H2,1-6H3/b9-8+/t20-,21-,22?,23+,24+,25-,26+,28+,29-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50176471

(5alpha,8alpha-epidioxy-(24S)-ethylcholesta-6,9(11)...)Show SMILES CC[C@H](\C=C\[C@@H](C)[C@H]1CC[C@@H]2[C@]1(C)CC=C1[C@@]3(C)CC[C@H](O)C[C@@]33OO[C@@]21C=C3)C(C)C |c:31,t:15| Show InChI InChI=1S/C29H44O3/c1-7-21(19(2)3)9-8-20(4)23-10-11-24-26(23,5)14-13-25-27(6)15-12-22(30)18-28(27)16-17-29(24,25)32-31-28/h8-9,13,16-17,19-24,30H,7,10-12,14-15,18H2,1-6H3/b9-8+/t20-,21-,22+,23-,24-,26-,27-,28-,29+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50176469

(CHEMBL202221 | haloxysterol A)Show SMILES CC[C@H](C[C@@H](O)[C@@H](C)[C@H]1CCC2C3CC=C4C[C@@H](O)C[C@H](O)[C@]4(C)C3CC[C@]12C)C(C)C |t:14| Show InChI InChI=1S/C29H50O3/c1-7-19(17(2)3)14-26(31)18(4)23-10-11-24-22-9-8-20-15-21(30)16-27(32)29(20,6)25(22)12-13-28(23,24)5/h8,17-19,21-27,30-32H,7,9-16H2,1-6H3/t18-,19+,21+,22?,23+,24?,25?,26+,27-,28+,29-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50176470

(24-ethyl-cholest-7-ene-3,5,6-triol | CHEMBL201951)Show SMILES CCC(CC[C@@H](C)[C@H]1CC[C@H]2C3=CC(O)C4(O)C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(C)C |t:11| Show InChI InChI=1S/C29H50O3/c1-7-20(18(2)3)9-8-19(4)23-10-11-24-22-16-26(31)29(32)17-21(30)12-15-28(29,6)25(22)13-14-27(23,24)5/h16,18-21,23-26,30-32H,7-15,17H2,1-6H3/t19-,20?,21+,23-,24+,25+,26?,27-,28-,29?/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50176472

(CHEMBL202496 | lawsaritol)Show SMILES CC[C@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CCC4=C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(C)C |t:14| Show InChI InChI=1S/C29H50O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h18-21,23-27,30H,7-17H2,1-6H3/t20-,21-,23+,24+,25-,26+,27+,28+,29-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50176467

(CHEMBL201253 | haloxysterol D)Show SMILES CC[C@H](C[C@@H](O)[C@@H](C)[C@H]1CCC2C3C[C@@H](O)[C@@]4(O)C[C@@H](O)C[C@H](O)[C@]4(C)C3CC[C@]12C)C(C)C Show InChI InChI=1S/C29H52O5/c1-7-18(16(2)3)12-24(31)17(4)21-8-9-22-20-14-26(33)29(34)15-19(30)13-25(32)28(29,6)23(20)10-11-27(21,22)5/h16-26,30-34H,7-15H2,1-6H3/t17-,18+,19-,20?,21+,22?,23?,24+,25-,26+,27+,28-,29-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50176465

(CHEMBL382961 | haloxysterol C)Show SMILES CC[C@H](C[C@@H](O)[C@@H](C)[C@H]1CCC2C3CCC4=CC(=O)CC[C@]4(C)C3=CC[C@]12C)C(C)C |c:25,t:15| Show InChI InChI=1S/C29H46O2/c1-7-20(18(2)3)16-27(31)19(4)24-10-11-25-23-9-8-21-17-22(30)12-14-28(21,5)26(23)13-15-29(24,25)6/h13,17-20,23-25,27,31H,7-12,14-16H2,1-6H3/t19-,20+,23?,24+,25?,27+,28-,29+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against BuChE from horse serum |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50176473

((24S)-ethylcholesta-7,9(11),22(E)-triene-3b-ol | C...)Show SMILES CC[C@H](\C=C\[C@@H](C)[C@H]1CC[C@H]2[C@@H]3C=CC4C[C@@H](O)CC[C@]4(C)C3=CC[C@]12C)C(C)C |c:12,24| Show InChI InChI=1S/C29H46O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h8-11,15,19-26,30H,7,12-14,16-18H2,1-6H3/b9-8+/t20-,21-,22?,23+,24+,25-,26+,28+,29-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50176471

(5alpha,8alpha-epidioxy-(24S)-ethylcholesta-6,9(11)...)Show SMILES CC[C@H](\C=C\[C@@H](C)[C@H]1CC[C@@H]2[C@]1(C)CC=C1[C@@]3(C)CC[C@H](O)C[C@@]33OO[C@@]21C=C3)C(C)C |c:31,t:15| Show InChI InChI=1S/C29H44O3/c1-7-21(19(2)3)9-8-20(4)23-10-11-24-26(23,5)14-13-25-27(6)15-12-22(30)18-28(27)16-17-29(24,25)32-31-28/h8-9,13,16-17,19-24,30H,7,10-12,14-15,18H2,1-6H3/b9-8+/t20-,21-,22+,23-,24-,26-,27-,28-,29+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibitory activity against AChE from electric eel |
Bioorg Med Chem Lett 16: 573-80 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.042
BindingDB Entry DOI: 10.7270/Q29P3170 |
More data for this
Ligand-Target Pair | |
Histamine N-methyltransferase
(Cavia porcellus) | BDBM50016564
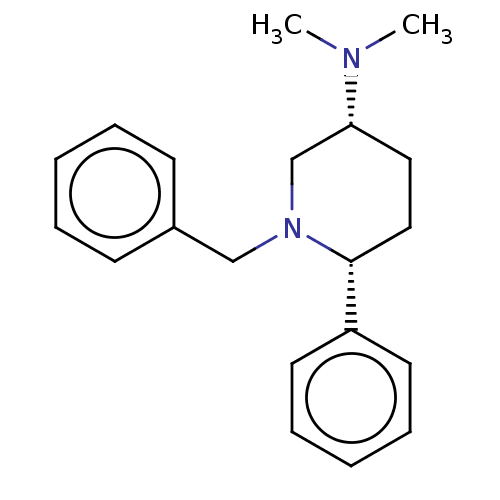
(CHEMBL3272448)Show SMILES CN(C)[C@@H]1CC[C@@H](N(Cc2ccccc2)C1)c1ccccc1 |r| Show InChI InChI=1S/C20H26N2/c1-21(2)19-13-14-20(18-11-7-4-8-12-18)22(16-19)15-17-9-5-3-6-10-17/h3-12,19-20H,13-16H2,1-2H3/t19-,20-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of histamine N-methyltransferase in guinea pig brain using S-adenosyl-L-methionine-14C as substrate by scintillation spectrophotometer |
J Med Chem 19: 117-22 (1976)
BindingDB Entry DOI: 10.7270/Q29W0H2G |
More data for this
Ligand-Target Pair | |
Histamine N-methyltransferase
(Cavia porcellus) | BDBM50016547
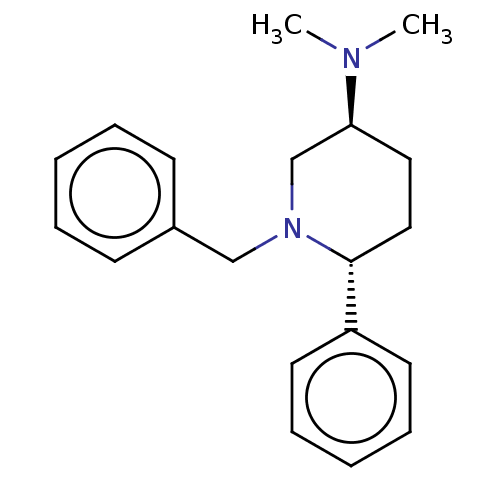
(CHEMBL3272447)Show SMILES CN(C)[C@H]1CC[C@@H](N(Cc2ccccc2)C1)c1ccccc1 |r| Show InChI InChI=1S/C20H26N2/c1-21(2)19-13-14-20(18-11-7-4-8-12-18)22(16-19)15-17-9-5-3-6-10-17/h3-12,19-20H,13-16H2,1-2H3/t19-,20+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of histamine N-methyltransferase in guinea pig brain using S-adenosyl-L-methionine-14C as substrate by scintillation spectrophotometer |
J Med Chem 19: 117-22 (1976)
BindingDB Entry DOI: 10.7270/Q29W0H2G |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073539
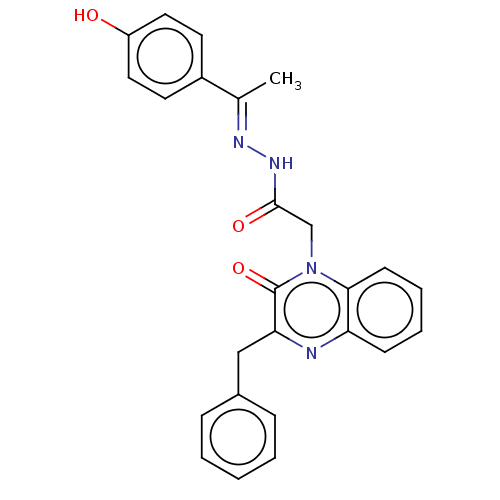
(CHEMBL3408926)Show SMILES C\C(=N/NC(=O)Cn1c2ccccc2nc(Cc2ccccc2)c1=O)c1ccc(O)cc1 Show InChI InChI=1S/C25H22N4O3/c1-17(19-11-13-20(30)14-12-19)27-28-24(31)16-29-23-10-6-5-9-21(23)26-22(25(29)32)15-18-7-3-2-4-8-18/h2-14,30H,15-16H2,1H3,(H,28,31)/b27-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073512

(CHEMBL3408911)Show InChI InChI=1S/C22H18N4O2/c27-17-12-10-16(11-13-17)22(28)26-25-21-20(14-15-6-2-1-3-7-15)23-18-8-4-5-9-19(18)24-21/h1-13,27H,14H2,(H,24,25)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073531

(CHEMBL3408918)Show SMILES O=C(Cn1c2ccccc2nc(Cc2ccccc2)c1=O)NNC(=O)C(Cc1ccccc1)NC(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C41H35N5O5/c47-38(25-46-37-22-12-11-21-34(37)42-36(40(46)49)24-28-15-5-2-6-16-28)44-45-39(48)35(23-27-13-3-1-4-14-27)43-41(50)51-26-33-31-19-9-7-17-29(31)30-18-8-10-20-32(30)33/h1-22,33,35H,23-26H2,(H,43,50)(H,44,47)(H,45,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073533

(CHEMBL3408920)Show SMILES O=C(Cn1c2ccccc2nc(Cc2ccccc2)c1=O)N\N=C\c1ccccc1 Show InChI InChI=1S/C24H20N4O2/c29-23(27-25-16-19-11-5-2-6-12-19)17-28-22-14-8-7-13-20(22)26-21(24(28)30)15-18-9-3-1-4-10-18/h1-14,16H,15,17H2,(H,27,29)/b25-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073532

(CHEMBL3408919)Show SMILES CC(C)(C)Oc1ccc(CC(NC(=O)OCC2c3ccccc3-c3ccccc23)C(=O)NNC(=O)Cn2c3ccccc3nc(Cc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C45H43N5O6/c1-45(2,3)56-31-23-21-30(22-24-31)25-38(47-44(54)55-28-36-34-17-9-7-15-32(34)33-16-8-10-18-35(33)36)42(52)49-48-41(51)27-50-40-20-12-11-19-37(40)46-39(43(50)53)26-29-13-5-4-6-14-29/h4-24,36,38H,25-28H2,1-3H3,(H,47,54)(H,48,51)(H,49,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073534

(CHEMBL3408921)Show SMILES Oc1ccc(\C=N\NC(=O)Cn2c3ccccc3nc(Cc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C24H20N4O3/c29-19-12-10-18(11-13-19)15-25-27-23(30)16-28-22-9-5-4-8-20(22)26-21(24(28)31)14-17-6-2-1-3-7-17/h1-13,15,29H,14,16H2,(H,27,30)/b25-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073535

(CHEMBL3408922)Show SMILES COc1ccc(\C=N\NC(=O)Cn2c3ccccc3nc(Cc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C25H22N4O3/c1-32-20-13-11-19(12-14-20)16-26-28-24(30)17-29-23-10-6-5-9-21(23)27-22(25(29)31)15-18-7-3-2-4-8-18/h2-14,16H,15,17H2,1H3,(H,28,30)/b26-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073540
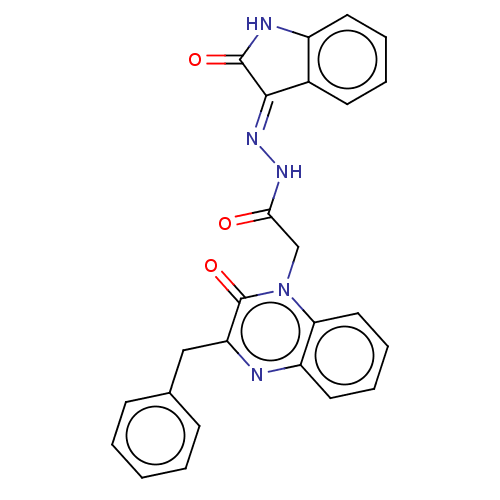
(CHEMBL3408927)Show SMILES O=C(Cn1c2ccccc2nc(Cc2ccccc2)c1=O)N\N=C1\C(=O)Nc2ccccc12 Show InChI InChI=1S/C25H19N5O3/c31-22(28-29-23-17-10-4-5-11-18(17)27-24(23)32)15-30-21-13-7-6-12-19(21)26-20(25(30)33)14-16-8-2-1-3-9-16/h1-13H,14-15H2,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073536

(CHEMBL3408923)Show SMILES Clc1ccc(\C=N\NC(=O)Cn2c3ccccc3nc(Cc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C24H19ClN4O2/c25-19-12-10-18(11-13-19)15-26-28-23(30)16-29-22-9-5-4-8-20(22)27-21(24(29)31)14-17-6-2-1-3-7-17/h1-13,15H,14,16H2,(H,28,30)/b26-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128258
BindingDB Entry DOI: 10.7270/Q2930XXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50512093

(CHEMBL4442101)Show InChI InChI=1S/C13H12N2O/c1-10-3-2-4-11(9-10)5-6-12-7-8-13(16)15-14-12/h2-9H,1H3,(H,15,16)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate preincubated with enzyme for 10 mins follo... |
Eur J Med Chem 171: 25-37 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.036
BindingDB Entry DOI: 10.7270/Q2222Z3M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate preincubated with enzyme for 10 mins follo... |
Eur J Med Chem 171: 25-37 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.036
BindingDB Entry DOI: 10.7270/Q2222Z3M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50512095
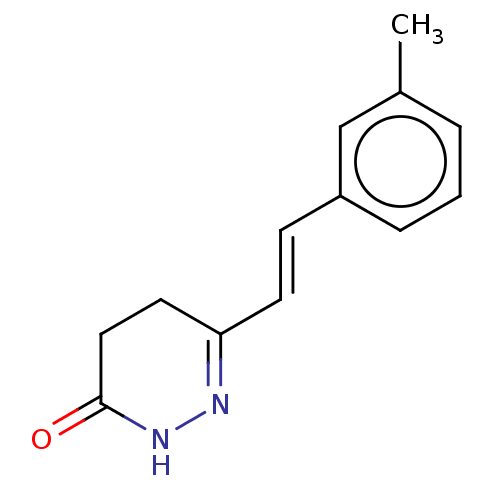
(CHEMBL4435643)Show InChI InChI=1S/C13H14N2O/c1-10-3-2-4-11(9-10)5-6-12-7-8-13(16)15-14-12/h2-6,9H,7-8H2,1H3,(H,15,16)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate preincubated with enzyme for 10 mins follo... |
Eur J Med Chem 171: 25-37 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.036
BindingDB Entry DOI: 10.7270/Q2222Z3M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50512097
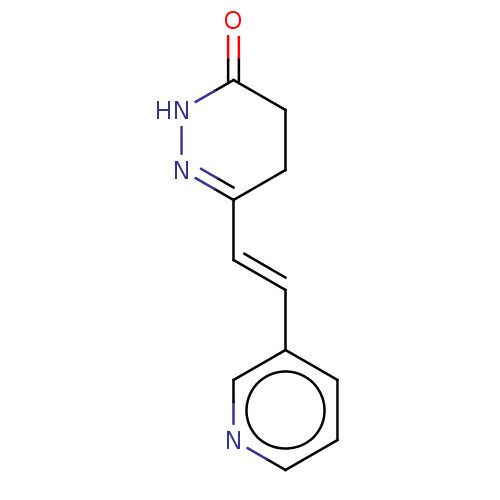
(CHEMBL4536976)Show InChI InChI=1S/C11H11N3O/c15-11-6-5-10(13-14-11)4-3-9-2-1-7-12-8-9/h1-4,7-8H,5-6H2,(H,14,15)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate preincubated with enzyme for 10 mins follo... |
Eur J Med Chem 171: 25-37 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.036
BindingDB Entry DOI: 10.7270/Q2222Z3M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50512102
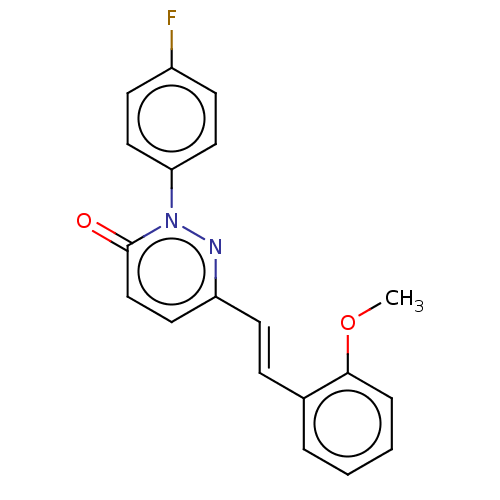
(CHEMBL4465207)Show InChI InChI=1S/C19H15FN2O2/c1-24-18-5-3-2-4-14(18)6-9-16-10-13-19(23)22(21-16)17-11-7-15(20)8-12-17/h2-13H,1H3/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate preincubated with enzyme for 10 mins follo... |
Eur J Med Chem 171: 25-37 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.036
BindingDB Entry DOI: 10.7270/Q2222Z3M |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50566726

(CHEMBL4868909)Show SMILES COc1cccc(c1)-c1cc(nc(N)c1C#N)-c1c(O)c2ccccc2oc1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDGFRbeta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128258
BindingDB Entry DOI: 10.7270/Q2930XXQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1/2/3/4
(Homo sapiens (Human)) | BDBM50566726

(CHEMBL4868909)Show SMILES COc1cccc(c1)-c1cc(nc(N)c1C#N)-c1c(O)c2ccccc2oc1=O | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128258
BindingDB Entry DOI: 10.7270/Q2930XXQ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073537

(CHEMBL3408924)Show SMILES Brc1ccc(\C=N\NC(=O)Cn2c3ccccc3nc(Cc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C24H19BrN4O2/c25-19-12-10-18(11-13-19)15-26-28-23(30)16-29-22-9-5-4-8-20(22)27-21(24(29)31)14-17-6-2-1-3-7-17/h1-13,15H,14,16H2,(H,28,30)/b26-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1/2/3/4
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128258
BindingDB Entry DOI: 10.7270/Q2930XXQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50566726

(CHEMBL4868909)Show SMILES COc1cccc(c1)-c1cc(nc(N)c1C#N)-c1c(O)c2ccccc2oc1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128258
BindingDB Entry DOI: 10.7270/Q2930XXQ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073510

(CHEMBL3408909)Show InChI InChI=1S/C22H17ClN4O/c23-17-12-10-16(11-13-17)22(28)27-26-21-20(14-15-6-2-1-3-7-15)24-18-8-4-5-9-19(18)25-21/h1-13H,14H2,(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50073543
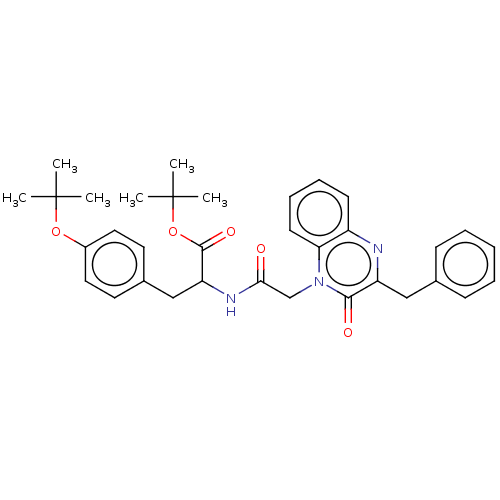
(CHEMBL3408930)Show SMILES CC(C)(C)OC(=O)C(Cc1ccc(OC(C)(C)C)cc1)NC(=O)Cn1c2ccccc2nc(Cc2ccccc2)c1=O Show InChI InChI=1S/C34H39N3O5/c1-33(2,3)41-25-18-16-24(17-19-25)21-28(32(40)42-34(4,5)6)36-30(38)22-37-29-15-11-10-14-26(29)35-27(31(37)39)20-23-12-8-7-9-13-23/h7-19,28H,20-22H2,1-6H3,(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAO-A preincubated for 60 mins before substrate addition by fluorimetric method |
Eur J Med Chem 93: 308-20 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.020
BindingDB Entry DOI: 10.7270/Q2VH5QJ9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50512098
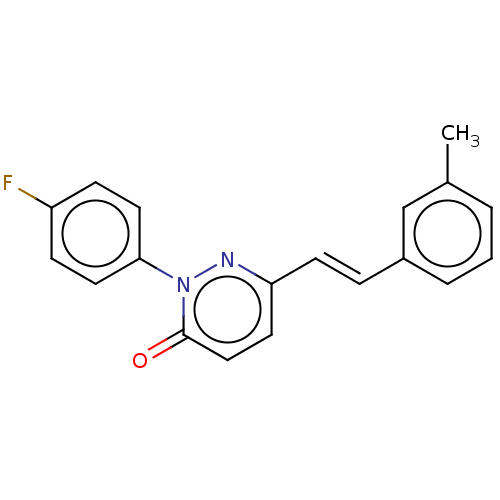
(CHEMBL4574755)Show InChI InChI=1S/C19H15FN2O/c1-14-3-2-4-15(13-14)5-8-17-9-12-19(23)22(21-17)18-10-6-16(20)7-11-18/h2-13H,1H3/b8-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate preincubated with enzyme for 10 mins follo... |
Eur J Med Chem 171: 25-37 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.036
BindingDB Entry DOI: 10.7270/Q2222Z3M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50537171
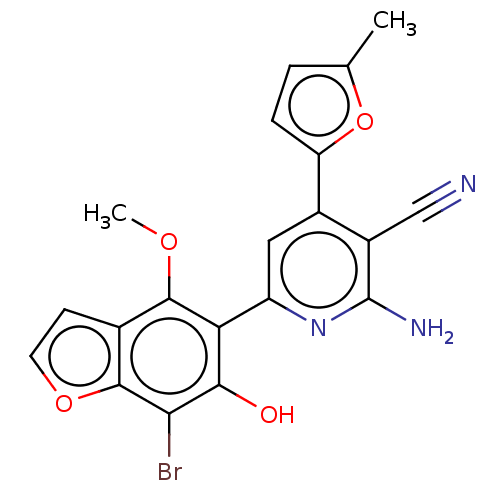
(CHEMBL4576947)Show SMILES COc1c(c(O)c(Br)c2occc12)-c1cc(-c2ccc(C)o2)c(C#N)c(N)n1 Show InChI InChI=1S/C20H14BrN3O4/c1-9-3-4-14(28-9)11-7-13(24-20(23)12(11)8-22)15-17(25)16(21)19-10(5-6-27-19)18(15)26-2/h3-7,25H,1-2H3,(H2,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAPK (unknown origin) using ATF-2 as substrate after 1 hr by ELISA |
Bioorg Med Chem 27: 1308-1319 (2019)
Article DOI: 10.1016/j.bmc.2019.02.027
BindingDB Entry DOI: 10.7270/Q2RB784N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data