

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433757 (CHEMBL2381443) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433756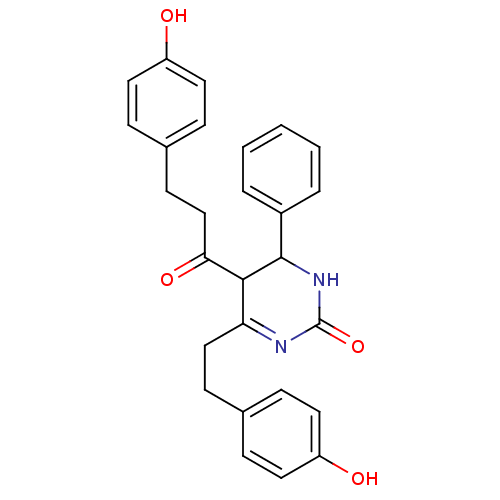 (CHEMBL2381442) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433755 (CHEMBL2381440) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433754 (LETESTUIANIN C | Tetrahydrobisdemethoxycurcumin) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433753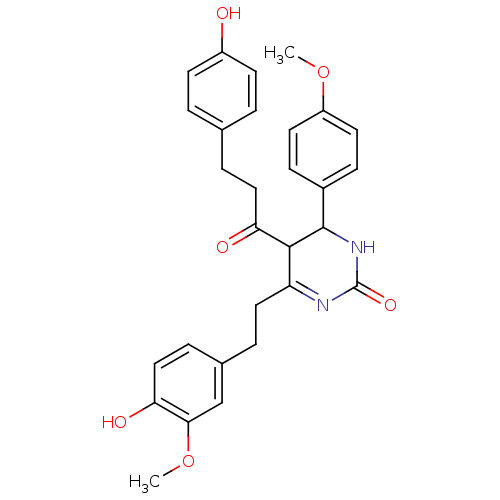 (CHEMBL2381441) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433752 (CHEMBL2381438) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433751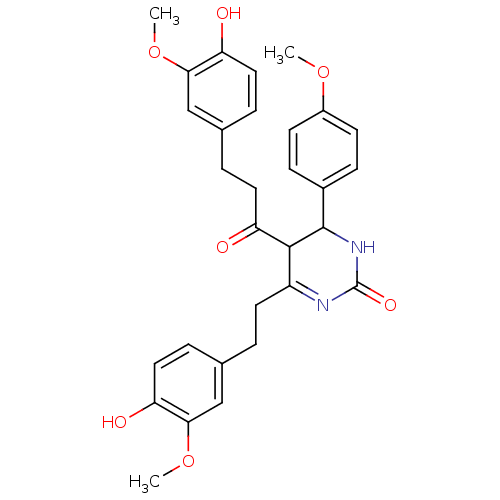 (CHEMBL2381434) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433750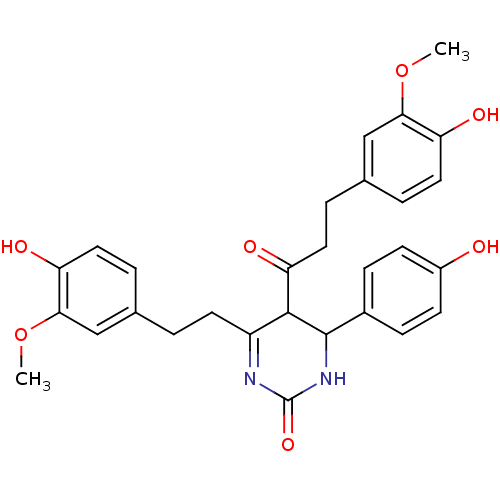 (CHEMBL2381433) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50059989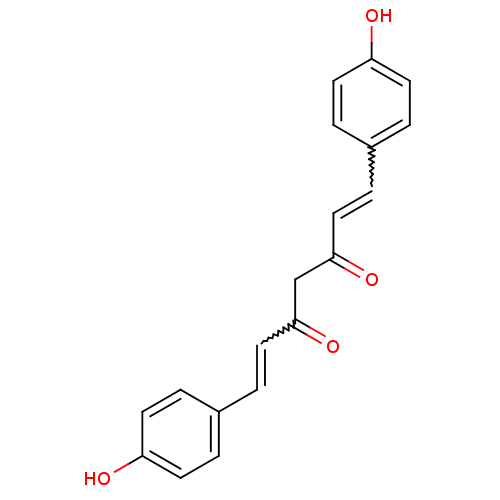 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433749 (TETRAHYDRODEMETHOXYCURCUMIN) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433748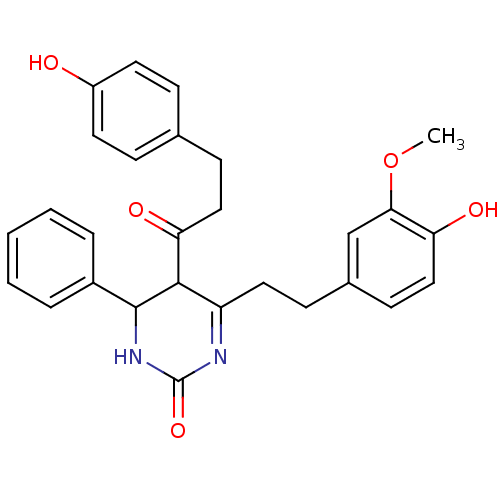 (CHEMBL2381439) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433747 (CHEMBL2381430) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50163744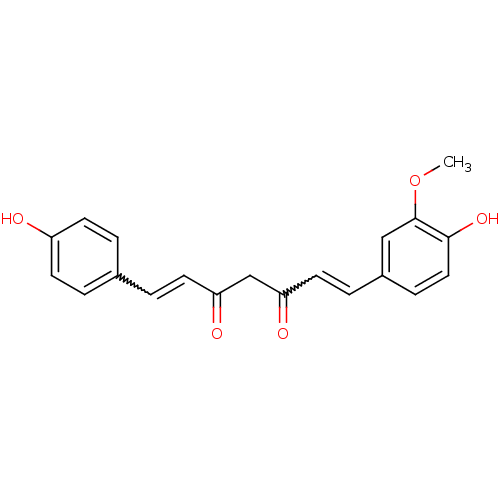 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50059985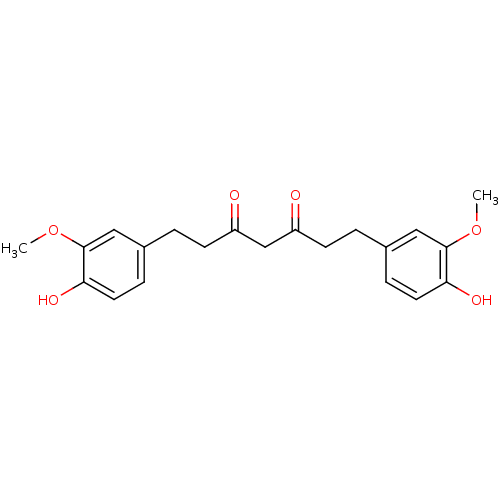 (1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-heptane-3,5-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433746 (CHEMBL2381437) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50067040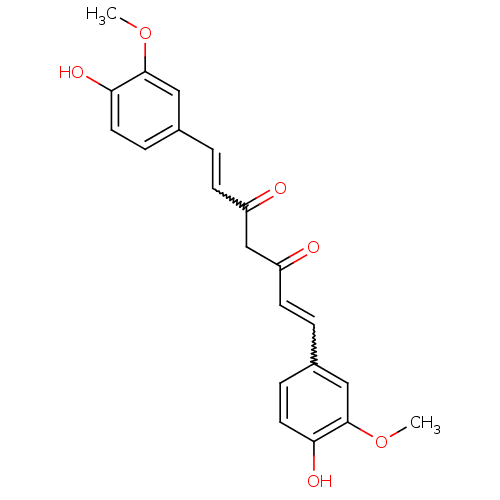 (((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||