 Found 40 hits with Last Name = 'akinaga' and Initial = 's'
Found 40 hits with Last Name = 'akinaga' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50275347
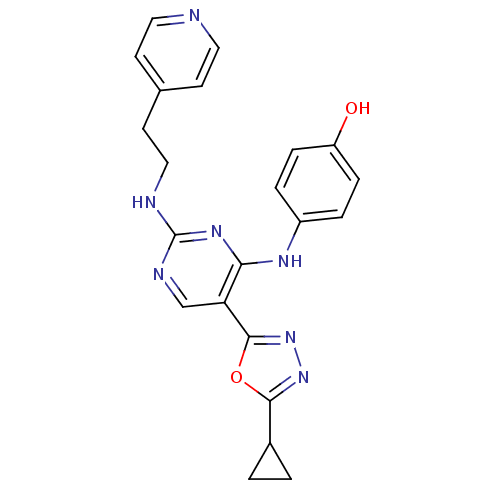
(4-(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-2-(2-(py...)Show SMILES Oc1ccc(Nc2nc(NCCc3ccncc3)ncc2-c2nnc(o2)C2CC2)cc1 Show InChI InChI=1S/C22H21N7O2/c30-17-5-3-16(4-6-17)26-19-18(21-29-28-20(31-21)15-1-2-15)13-25-22(27-19)24-12-9-14-7-10-23-11-8-14/h3-8,10-11,13,15,30H,1-2,9,12H2,(H2,24,25,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50276003
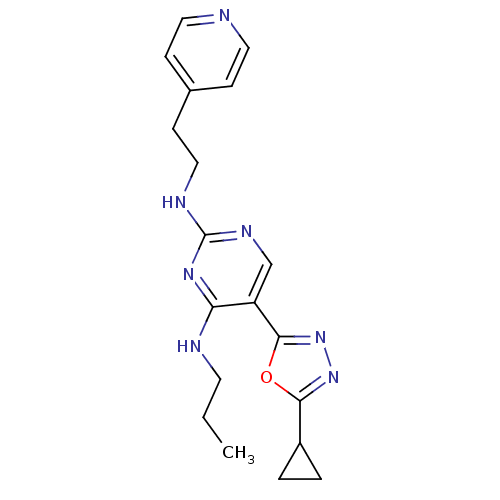
(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-N4-propyl-N...)Show InChI InChI=1S/C19H23N7O/c1-2-8-21-16-15(18-26-25-17(27-18)14-3-4-14)12-23-19(24-16)22-11-7-13-5-9-20-10-6-13/h5-6,9-10,12,14H,2-4,7-8,11H2,1H3,(H2,21,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50024270
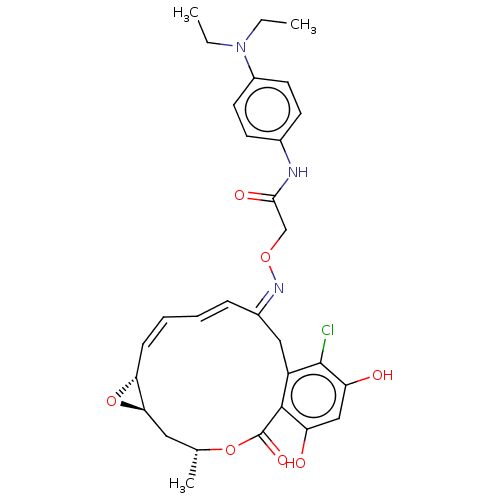
(CHEMBL85612)Show SMILES [H][C@@]12C[C@@H](C)OC(=O)c3c(O)cc(O)c(Cl)c3C\C(\C=C\C=C/[C@@]1([H])O2)=N\OCC(=O)Nc1ccc(cc1)N(CC)CC |c:22,t:20| Show InChI InChI=1S/C30H34ClN3O7/c1-4-34(5-2)21-12-10-19(11-13-21)32-27(37)17-39-33-20-8-6-7-9-25-26(41-25)14-18(3)40-30(38)28-22(15-20)29(31)24(36)16-23(28)35/h6-13,16,18,25-26,35-36H,4-5,14-15,17H2,1-3H3,(H,32,37)/b8-6+,9-7-,33-20+/t18-,25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128843
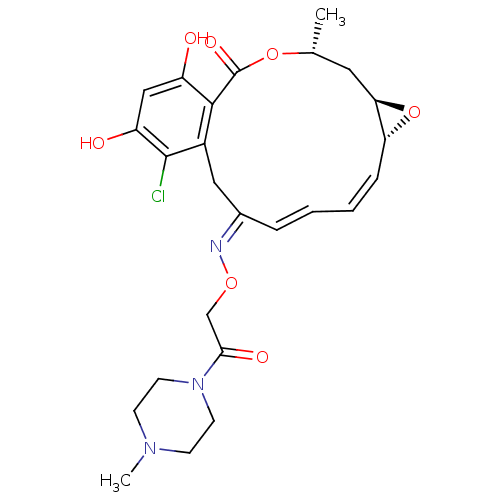
(16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)N1CCN(C)CC1 |c:7,t:9| Show InChI InChI=1S/C25H30ClN3O7/c1-15-11-21-20(36-21)6-4-3-5-16(27-34-14-22(32)29-9-7-28(2)8-10-29)12-17-23(25(33)35-15)18(30)13-19(31)24(17)26/h3-6,13,15,20-21,30-31H,7-12,14H2,1-2H3/b5-3+,6-4-,27-16+/t15-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128852

(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)NC1CCCC1 |c:7,t:9| Show InChI InChI=1S/C25H29ClN2O7/c1-14-10-21-20(35-21)9-5-4-8-16(28-33-13-22(31)27-15-6-2-3-7-15)11-17-23(25(32)34-14)18(29)12-19(30)24(17)26/h4-5,8-9,12,14-15,20-21,29-30H,2-3,6-7,10-11,13H2,1H3,(H,27,31)/b8-4+,9-5-,28-16+/t14-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128851

(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)NCC1CCCCC1 |c:7,t:9| Show InChI InChI=1S/C27H33ClN2O7/c1-16-11-23-22(37-23)10-6-5-9-18(30-35-15-24(33)29-14-17-7-3-2-4-8-17)12-19-25(27(34)36-16)20(31)13-21(32)26(19)28/h5-6,9-10,13,16-17,22-23,31-32H,2-4,7-8,11-12,14-15H2,1H3,(H,29,33)/b9-5+,10-6-,30-18+/t16-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128859
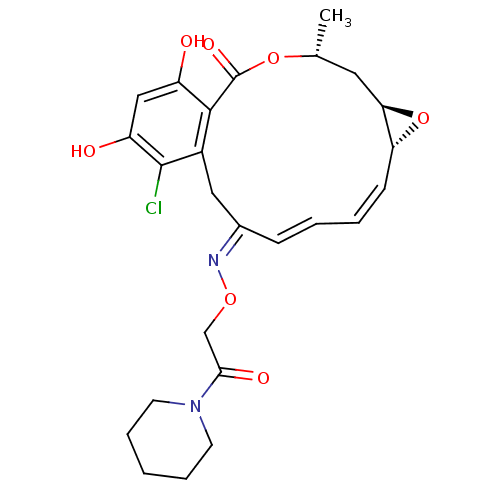
(16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)N1CCCCC1 |c:7,t:9| Show InChI InChI=1S/C25H29ClN2O7/c1-15-11-21-20(35-21)8-4-3-7-16(27-33-14-22(31)28-9-5-2-6-10-28)12-17-23(25(32)34-15)18(29)13-19(30)24(17)26/h3-4,7-8,13,15,20-21,29-30H,2,5-6,9-12,14H2,1H3/b7-3+,8-4-,27-16+/t15-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50275348
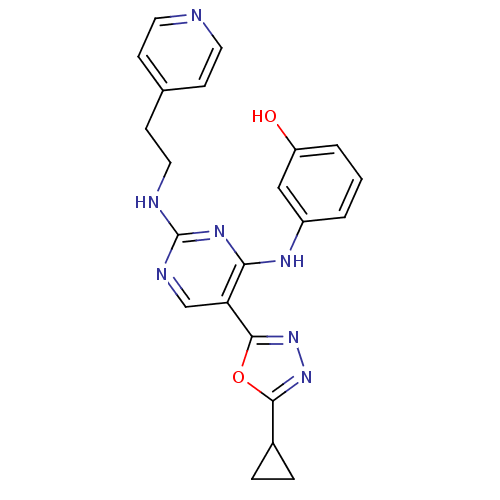
(3-(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-2-(2-(py...)Show SMILES Oc1cccc(Nc2nc(NCCc3ccncc3)ncc2-c2nnc(o2)C2CC2)c1 Show InChI InChI=1S/C22H21N7O2/c30-17-3-1-2-16(12-17)26-19-18(21-29-28-20(31-21)15-4-5-15)13-25-22(27-19)24-11-8-14-6-9-23-10-7-14/h1-3,6-7,9-10,12-13,15,30H,4-5,8,11H2,(H2,24,25,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50276053
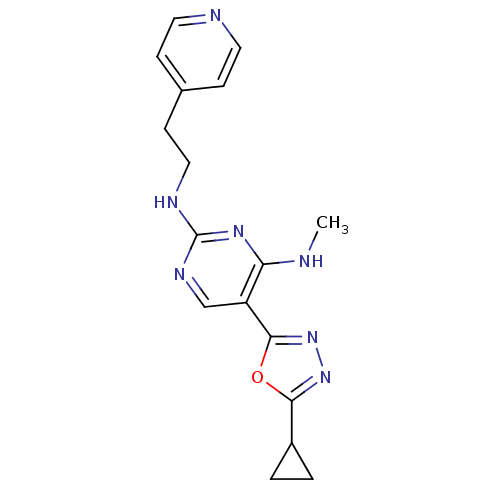
(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-N4-methyl-N...)Show InChI InChI=1S/C17H19N7O/c1-18-14-13(16-24-23-15(25-16)12-2-3-12)10-21-17(22-14)20-9-6-11-4-7-19-8-5-11/h4-5,7-8,10,12H,2-3,6,9H2,1H3,(H2,18,20,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128846
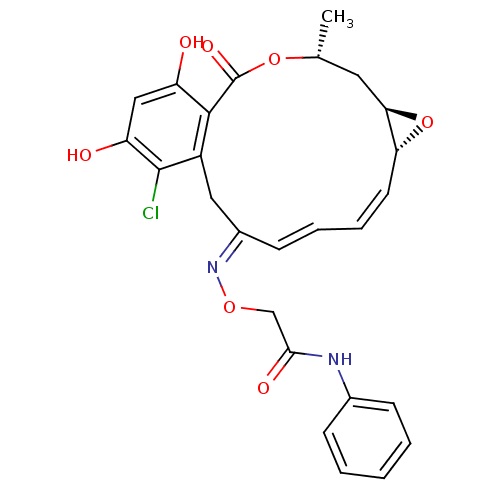
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)Nc1ccccc1 |c:7,t:9| Show InChI InChI=1S/C26H25ClN2O7/c1-15-11-22-21(36-22)10-6-5-9-17(29-34-14-23(32)28-16-7-3-2-4-8-16)12-18-24(26(33)35-15)19(30)13-20(31)25(18)27/h2-10,13,15,21-22,30-31H,11-12,14H2,1H3,(H,28,32)/b9-5+,10-6-,29-17+/t15-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054109

(CHEMBL3319301)Show SMILES CC(C)(C)C(=O)NC1=NN(C(=O)C(C)(C)C)C(CNS(C)(=O)=O)(S1)c1ccccc1 |t:7| Show InChI InChI=1S/C20H30N4O4S2/c1-18(2,3)15(25)22-17-23-24(16(26)19(4,5)6)20(29-17,13-21-30(7,27)28)14-11-9-8-10-12-14/h8-12,21H,13H2,1-7H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50276056
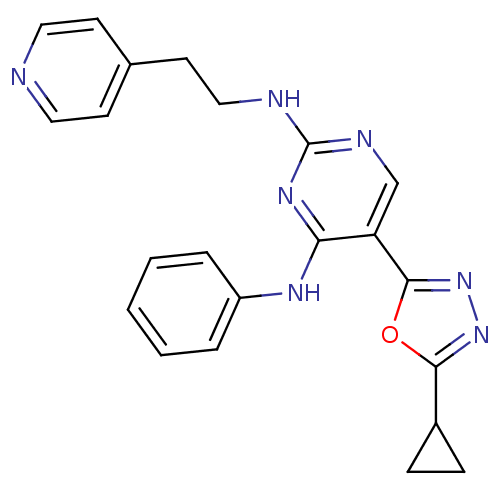
(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-N4-phenyl-N...)Show SMILES C(Cc1ccncc1)Nc1ncc(-c2nnc(o2)C2CC2)c(Nc2ccccc2)n1 Show InChI InChI=1S/C22H21N7O/c1-2-4-17(5-3-1)26-19-18(21-29-28-20(30-21)16-6-7-16)14-25-22(27-19)24-13-10-15-8-11-23-12-9-15/h1-5,8-9,11-12,14,16H,6-7,10,13H2,(H2,24,25,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128850
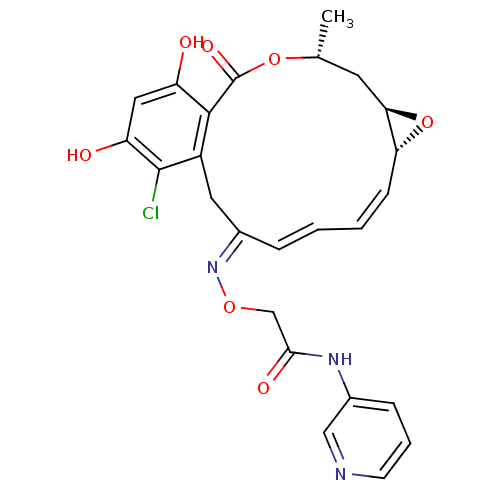
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)Nc1cccnc1 |c:7,t:9| Show InChI InChI=1S/C25H24ClN3O7/c1-14-9-21-20(36-21)7-3-2-5-15(29-34-13-22(32)28-16-6-4-8-27-12-16)10-17-23(25(33)35-14)18(30)11-19(31)24(17)26/h2-8,11-12,14,20-21,30-31H,9-10,13H2,1H3,(H,28,32)/b5-2+,7-3-,29-15+/t14-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128847

(10-Oxime radicicol | 16-Chloro-17,19-dihydroxy-4-m...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2C=CCC=C(Cc2c(Cl)c(O)cc(O)c2C(=O)O1)N=O |w:7.8,9.9| Show InChI InChI=1S/C18H18ClNO6/c1-9-6-15-14(26-15)5-3-2-4-10(20-24)7-11-16(18(23)25-9)12(21)8-13(22)17(11)19/h3-5,8-9,14-15,21-22H,2,6-7H2,1H3/t9-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50276054
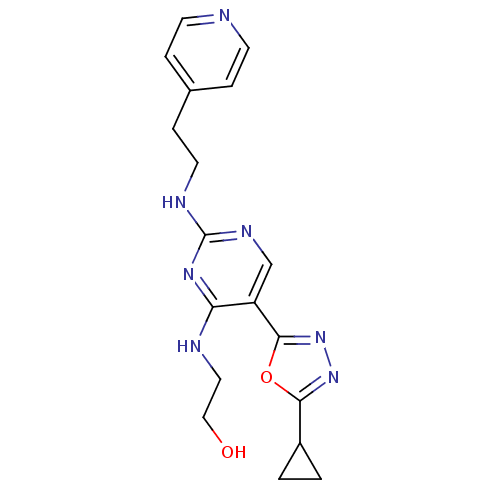
(2-(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-2-(2-(py...)Show InChI InChI=1S/C18H21N7O2/c26-10-9-20-15-14(17-25-24-16(27-17)13-1-2-13)11-22-18(23-15)21-8-5-12-3-6-19-7-4-12/h3-4,6-7,11,13,26H,1-2,5,8-10H2,(H2,20,21,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128857
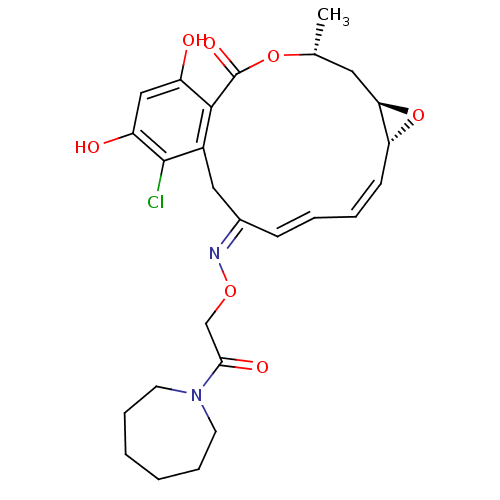
(16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)N1CCCCCC1 |c:7,t:9| Show InChI InChI=1S/C26H31ClN2O7/c1-16-12-22-21(36-22)9-5-4-8-17(28-34-15-23(32)29-10-6-2-3-7-11-29)13-18-24(26(33)35-16)19(30)14-20(31)25(18)27/h4-5,8-9,14,16,21-22,30-31H,2-3,6-7,10-13,15H2,1H3/b8-4+,9-5-,28-17+/t16-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128853
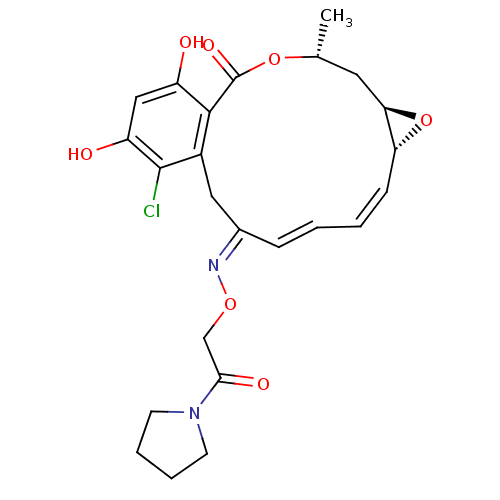
(16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)N1CCCC1 |c:7,t:9| Show InChI InChI=1S/C24H27ClN2O7/c1-14-10-20-19(34-20)7-3-2-6-15(26-32-13-21(30)27-8-4-5-9-27)11-16-22(24(31)33-14)17(28)12-18(29)23(16)25/h2-3,6-7,12,14,19-20,28-29H,4-5,8-11,13H2,1H3/b6-2+,7-3-,26-15+/t14-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50275349
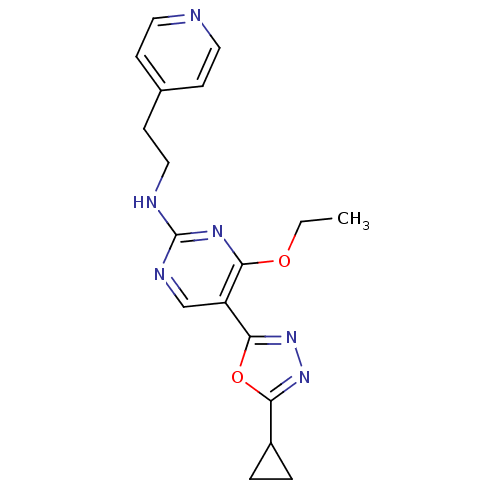
(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-4-ethoxy-N-...)Show InChI InChI=1S/C18H20N6O2/c1-2-25-16-14(17-24-23-15(26-17)13-3-4-13)11-21-18(22-16)20-10-7-12-5-8-19-9-6-12/h5-6,8-9,11,13H,2-4,7,10H2,1H3,(H,20,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50275346
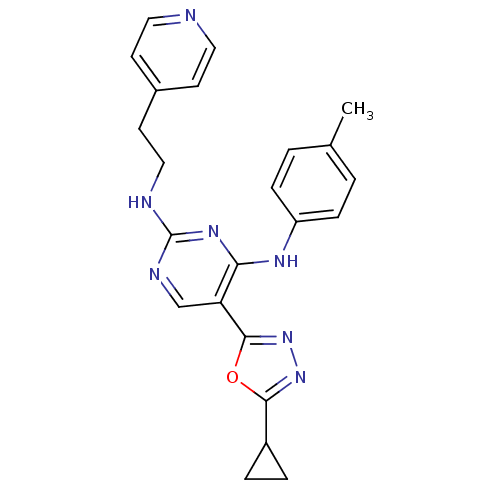
(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-N2-(2-(pyri...)Show SMILES Cc1ccc(Nc2nc(NCCc3ccncc3)ncc2-c2nnc(o2)C2CC2)cc1 Show InChI InChI=1S/C23H23N7O/c1-15-2-6-18(7-3-15)27-20-19(22-30-29-21(31-22)17-4-5-17)14-26-23(28-20)25-13-10-16-8-11-24-12-9-16/h2-3,6-9,11-12,14,17H,4-5,10,13H2,1H3,(H2,25,26,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054192

(CHEMBL3319304)Show SMILES CC(C)C(=O)N1N=C(NC(=O)C(C)(C)C)SC1(CCNS(C)(=O)=O)c1ccccc1 |t:6| Show InChI InChI=1S/C20H30N4O4S2/c1-14(2)16(25)24-20(12-13-21-30(6,27)28,15-10-8-7-9-11-15)29-18(23-24)22-17(26)19(3,4)5/h7-11,14,21H,12-13H2,1-6H3,(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054192

(CHEMBL3319304)Show SMILES CC(C)C(=O)N1N=C(NC(=O)C(C)(C)C)SC1(CCNS(C)(=O)=O)c1ccccc1 |t:6| Show InChI InChI=1S/C20H30N4O4S2/c1-14(2)16(25)24-20(12-13-21-30(6,27)28,15-10-8-7-9-11-15)29-18(23-24)22-17(26)19(3,4)5/h7-11,14,21H,12-13H2,1-6H3,(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054187
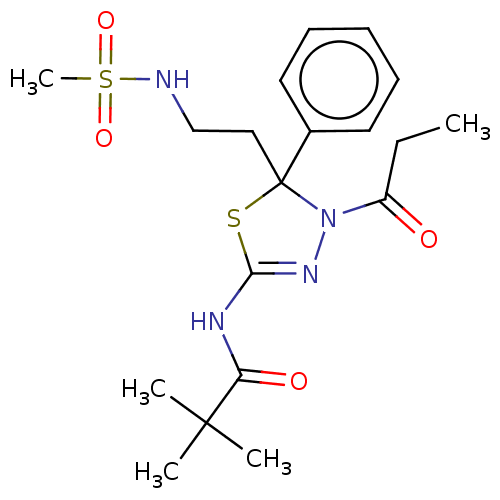
(CHEMBL3319303)Show SMILES CCC(=O)N1N=C(NC(=O)C(C)(C)C)SC1(CCNS(C)(=O)=O)c1ccccc1 |t:5| Show InChI InChI=1S/C19H28N4O4S2/c1-6-15(24)23-19(12-13-20-29(5,26)27,14-10-8-7-9-11-14)28-17(22-23)21-16(25)18(2,3)4/h7-11,20H,6,12-13H2,1-5H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054187

(CHEMBL3319303)Show SMILES CCC(=O)N1N=C(NC(=O)C(C)(C)C)SC1(CCNS(C)(=O)=O)c1ccccc1 |t:5| Show InChI InChI=1S/C19H28N4O4S2/c1-6-15(24)23-19(12-13-20-29(5,26)27,14-10-8-7-9-11-14)28-17(22-23)21-16(25)18(2,3)4/h7-11,20H,6,12-13H2,1-5H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054136

(CHEMBL3319302)Show SMILES CC(C)(C)C(=O)NC1=NN(C(=O)C(C)(C)C)C(CCNS(C)(=O)=O)(S1)c1ccccc1 |t:7| Show InChI InChI=1S/C21H32N4O4S2/c1-19(2,3)16(26)23-18-24-25(17(27)20(4,5)6)21(30-18,13-14-22-31(7,28)29)15-11-9-8-10-12-15/h8-12,22H,13-14H2,1-7H3,(H,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50276057
(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-N2-(2-(pyri...)Show SMILES Cc1ccccc1Nc1nc(NCCc2ccncc2)ncc1-c1nnc(o1)C1CC1 Show InChI InChI=1S/C23H23N7O/c1-15-4-2-3-5-19(15)27-20-18(22-30-29-21(31-22)17-6-7-17)14-26-23(28-20)25-13-10-16-8-11-24-12-9-16/h2-5,8-9,11-12,14,17H,6-7,10,13H2,1H3,(H2,25,26,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128845
(16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)N1CCOCC1 |c:7,t:9| Show InChI InChI=1S/C24H27ClN2O8/c1-14-10-20-19(35-20)5-3-2-4-15(26-33-13-21(30)27-6-8-32-9-7-27)11-16-22(24(31)34-14)17(28)12-18(29)23(16)25/h2-5,12,14,19-20,28-29H,6-11,13H2,1H3/b4-2+,5-3-,26-15+/t14-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50073099
((9E,11E)-(4R,6R,8R)-16-chloro-17,19-dihydroxy-4-me...)Show SMILES C[C@H]1C[C@H]2O[C@H]2C=CC=CC(=O)Cc2c(Cl)c(O)cc(O)c2C(=O)O1 |w:7.8,9.10| Show InChI InChI=1S/C18H17ClO6/c1-9-6-15-14(25-15)5-3-2-4-10(20)7-11-16(18(23)24-9)12(21)8-13(22)17(11)19/h2-5,8-9,14-15,21-22H,6-7H2,1H3/t9-,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128854
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)NC1CCCCC1 |c:7,t:9| Show InChI InChI=1S/C26H31ClN2O7/c1-15-11-22-21(36-22)10-6-5-9-17(29-34-14-23(32)28-16-7-3-2-4-8-16)12-18-24(26(33)35-15)19(30)13-20(31)25(18)27/h5-6,9-10,13,15-16,21-22,30-31H,2-4,7-8,11-12,14H2,1H3,(H,28,32)/b9-5+,10-6-,29-17+/t15-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128842
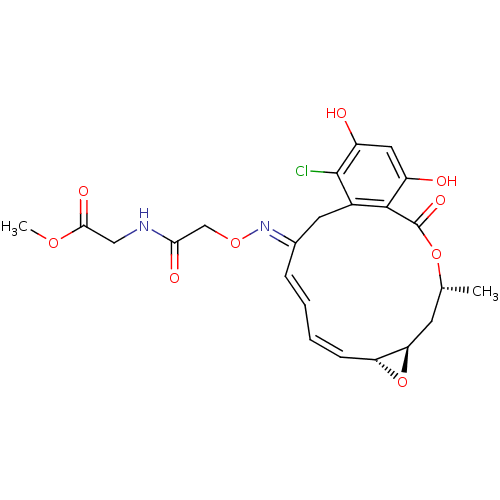
(CHEMBL84714 | [2-(16-Chloro-17,19-dihydroxy-4-meth...)Show SMILES COC(=O)CNC(=O)CO\N=C1\Cc2c(Cl)c(O)cc(O)c2C(=O)O[C@H](C)C[C@H]2O[C@@H]2/C=C\C=C\1 |c:33,t:35| Show InChI InChI=1S/C23H25ClN2O9/c1-12-7-18-17(35-18)6-4-3-5-13(26-33-11-19(29)25-10-20(30)32-2)8-14-21(23(31)34-12)15(27)9-16(28)22(14)24/h3-6,9,12,17-18,27-28H,7-8,10-11H2,1-2H3,(H,25,29)/b5-3+,6-4-,26-13+/t12-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50275746
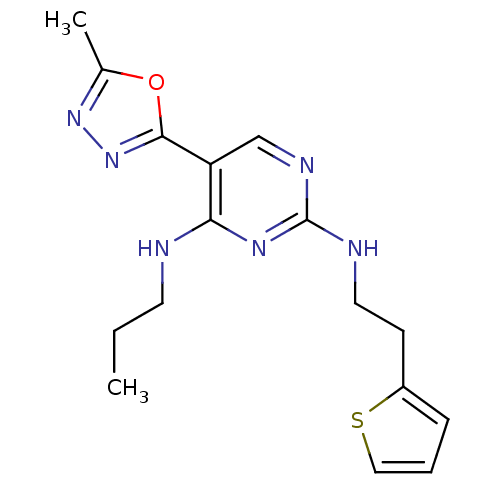
(5-(5-methyl-1,3,4-oxadiazol-2-yl)-N4-propyl-N2-(2-...)Show InChI InChI=1S/C16H20N6OS/c1-3-7-17-14-13(15-22-21-11(2)23-15)10-19-16(20-14)18-8-6-12-5-4-9-24-12/h4-5,9-10H,3,6-8H2,1-2H3,(H2,17,18,19,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128860
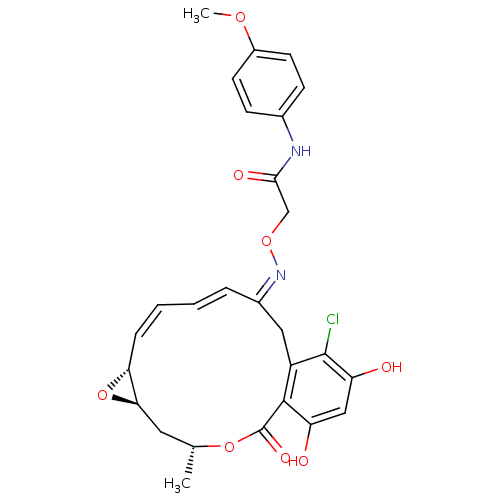
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES COc1ccc(NC(=O)CO\N=C2\Cc3c(Cl)c(O)cc(O)c3C(=O)O[C@H](C)C[C@H]3O[C@@H]3/C=C\C=C\2)cc1 |c:34,t:36| Show InChI InChI=1S/C27H27ClN2O8/c1-15-11-23-22(38-23)6-4-3-5-17(12-19-25(27(34)37-15)20(31)13-21(32)26(19)28)30-36-14-24(33)29-16-7-9-18(35-2)10-8-16/h3-10,13,15,22-23,31-32H,11-12,14H2,1-2H3,(H,29,33)/b5-3+,6-4-,30-17+/t15-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50276055
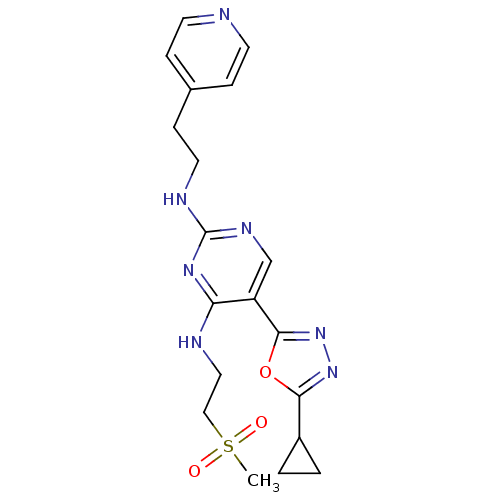
(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-N4-(2-(meth...)Show SMILES CS(=O)(=O)CCNc1nc(NCCc2ccncc2)ncc1-c1nnc(o1)C1CC1 Show InChI InChI=1S/C19H23N7O3S/c1-30(27,28)11-10-21-16-15(18-26-25-17(29-18)14-2-3-14)12-23-19(24-16)22-9-6-13-4-7-20-8-5-13/h4-5,7-8,12,14H,2-3,6,9-11H2,1H3,(H2,21,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 515 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 5472-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.031
BindingDB Entry DOI: 10.7270/Q2WD40DQ |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054108
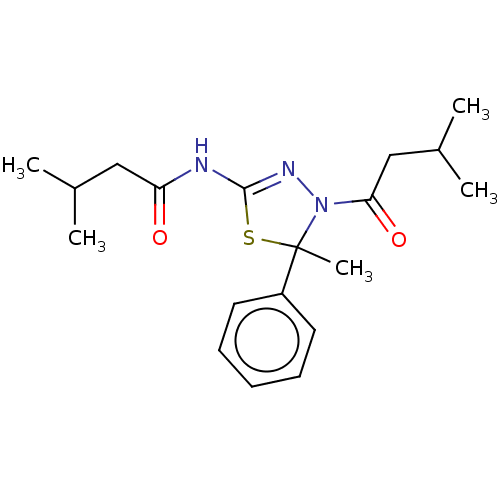
(CHEMBL3356912)Show SMILES CC(C)CC(=O)NC1=NN(C(=O)CC(C)C)C(C)(S1)c1ccccc1 |t:7| Show InChI InChI=1S/C19H27N3O2S/c1-13(2)11-16(23)20-18-21-22(17(24)12-14(3)4)19(5,25-18)15-9-7-6-8-10-15/h6-10,13-14H,11-12H2,1-5H3,(H,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128861
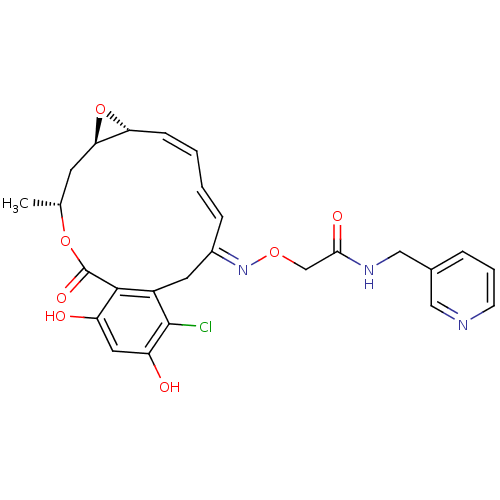
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)NCc1cccnc1 |c:7,t:9| Show InChI InChI=1S/C26H26ClN3O7/c1-15-9-22-21(37-22)7-3-2-6-17(30-35-14-23(33)29-13-16-5-4-8-28-12-16)10-18-24(26(34)36-15)19(31)11-20(32)25(18)27/h2-8,11-12,15,21-22,31-32H,9-10,13-14H2,1H3,(H,29,33)/b6-2+,7-3-,30-17+/t15-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128863
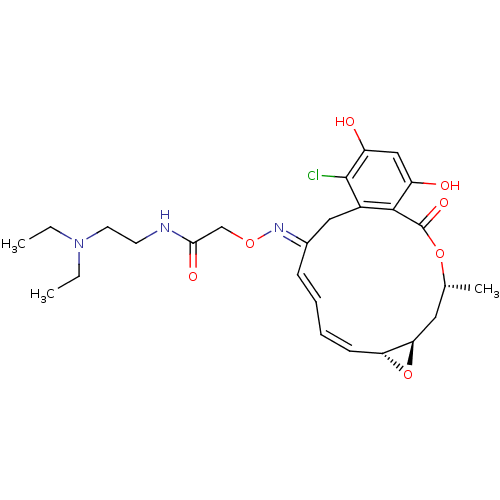
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES CCN(CC)CCNC(=O)CO\N=C1\Cc2c(Cl)c(O)cc(O)c2C(=O)O[C@H](C)C[C@H]2O[C@@H]2/C=C\C=C\1 |c:35,t:37| Show InChI InChI=1S/C26H34ClN3O7/c1-4-30(5-2)11-10-28-23(33)15-35-29-17-8-6-7-9-21-22(37-21)12-16(3)36-26(34)24-18(13-17)25(27)20(32)14-19(24)31/h6-9,14,16,21-22,31-32H,4-5,10-13,15H2,1-3H3,(H,28,33)/b8-6+,9-7-,29-17+/t16-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128864
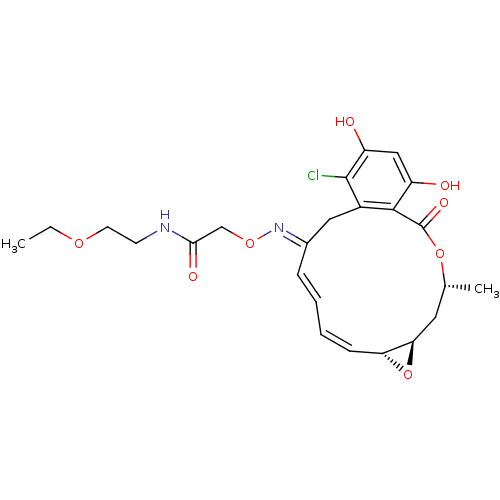
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES CCOCCNC(=O)CO\N=C1\Cc2c(Cl)c(O)cc(O)c2C(=O)O[C@H](C)C[C@H]2O[C@@H]2/C=C\C=C\1 |c:33,t:35| Show InChI InChI=1S/C24H29ClN2O8/c1-3-32-9-8-26-21(30)13-33-27-15-6-4-5-7-19-20(35-19)10-14(2)34-24(31)22-16(11-15)23(25)18(29)12-17(22)28/h4-7,12,14,19-20,28-29H,3,8-11,13H2,1-2H3,(H,26,30)/b6-4+,7-5-,27-15+/t14-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128848
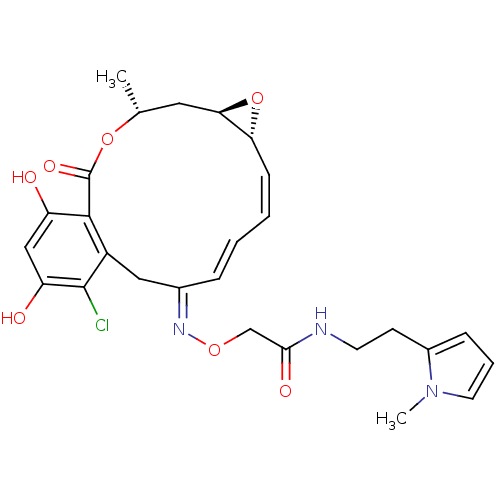
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)NCCc1cccn1C |c:7,t:9| Show InChI InChI=1S/C27H30ClN3O7/c1-16-12-23-22(38-23)8-4-3-6-17(13-19-25(27(35)37-16)20(32)14-21(33)26(19)28)30-36-15-24(34)29-10-9-18-7-5-11-31(18)2/h3-8,11,14,16,22-23,32-33H,9-10,12-13,15H2,1-2H3,(H,29,34)/b6-3+,8-4-,30-17+/t16-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128844
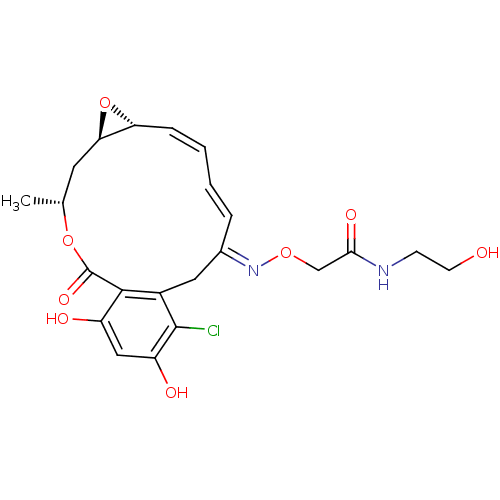
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)NCCO |c:7,t:9| Show InChI InChI=1S/C22H25ClN2O8/c1-12-8-18-17(33-18)5-3-2-4-13(25-31-11-19(29)24-6-7-26)9-14-20(22(30)32-12)15(27)10-16(28)21(14)23/h2-5,10,12,17-18,26-28H,6-9,11H2,1H3,(H,24,29)/b4-2+,5-3-,25-13+/t12-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Rattus norvegicus) | BDBM50128858
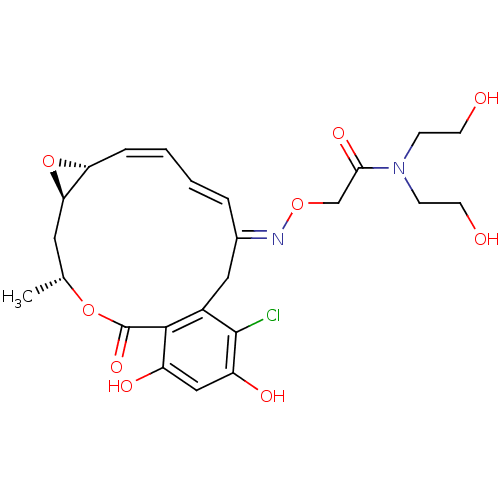
(2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...)Show SMILES C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)N(CCO)CCO |c:7,t:9| Show InChI InChI=1S/C24H29ClN2O9/c1-14-10-20-19(36-20)5-3-2-4-15(26-34-13-21(32)27(6-8-28)7-9-29)11-16-22(24(33)35-14)17(30)12-18(31)23(16)25/h2-5,12,14,19-20,28-31H,6-11,13H2,1H3/b4-2+,5-3-,26-15+/t14-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure |
J Med Chem 46: 2534-41 (2003)
Article DOI: 10.1021/jm030110r
BindingDB Entry DOI: 10.7270/Q2H41QTG |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50431886

(CHEMBL1413473)Show InChI InChI=1S/C13H15N3O2S/c1-9(17)14-12-15-16(10(2)18)13(3,19-12)11-7-5-4-6-8-11/h4-8H,1-3H3,(H,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data