

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197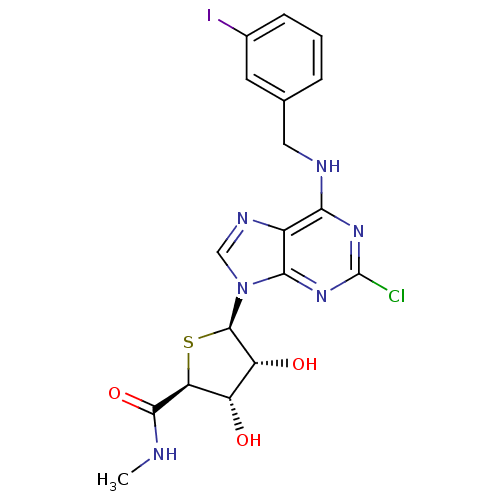 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50293031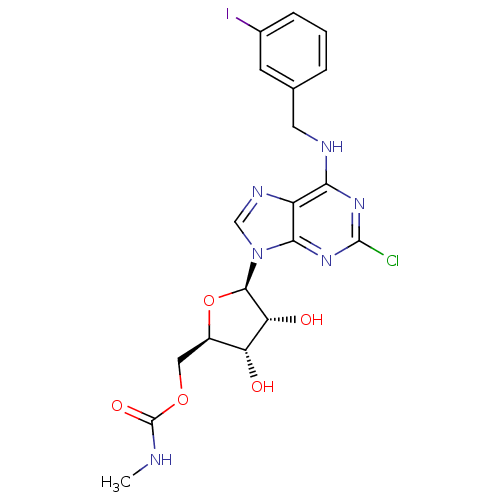 (2-chloro-N6-(3-iodobenzyl)-5'-N-methylcarbamoylade...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50214981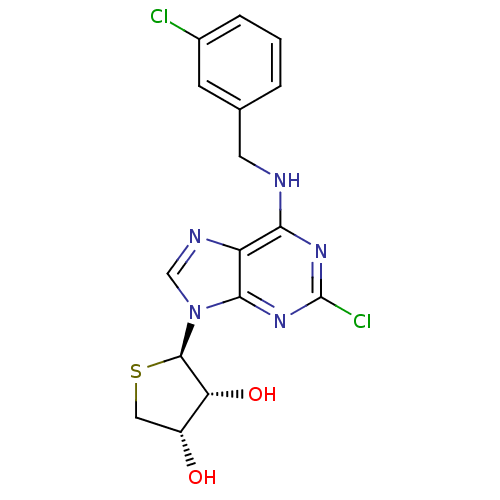 ((2R,3R,4S)-2-(2-chloro-6-(3-chlorobenzylamino)-9H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells at 10 uM | ACS Med Chem Lett 9: 516-520 (2010) Article DOI: 10.1021/ml1001823 BindingDB Entry DOI: 10.7270/Q24T6KCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50580163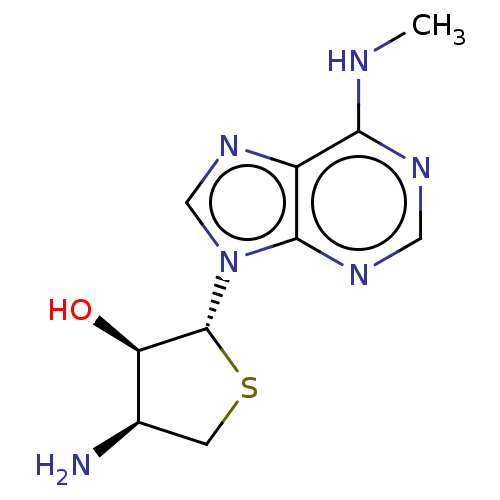 (CHEMBL5075606) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in CHO cell membrane incubated for 60 mins by gamma counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00239 BindingDB Entry DOI: 10.7270/Q2PC367C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50339076 ((2R,3R,4S)-2-(6-amino-2-(hex-1-ynyl)-9H-purin-9-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor in HEK293 cells | ACS Med Chem Lett 9: 516-520 (2010) Article DOI: 10.1021/ml1001823 BindingDB Entry DOI: 10.7270/Q24T6KCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50339076 ((2R,3R,4S)-2-(6-amino-2-(hex-1-ynyl)-9H-purin-9-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50339076 ((2R,3R,4S)-2-(6-amino-2-(hex-1-ynyl)-9H-purin-9-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells at 10 uM | ACS Med Chem Lett 9: 516-520 (2010) Article DOI: 10.1021/ml1001823 BindingDB Entry DOI: 10.7270/Q24T6KCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50339077 ((2R,3R,4S)-2-(6-amino-2-((E)-hex-1-enyl)-9H-purin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells at 10 uM | ACS Med Chem Lett 9: 516-520 (2010) Article DOI: 10.1021/ml1001823 BindingDB Entry DOI: 10.7270/Q24T6KCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50339078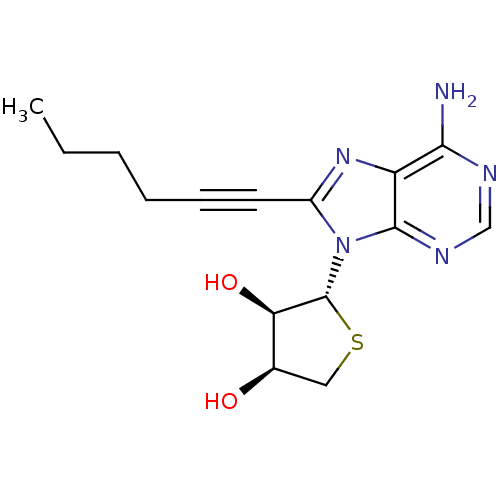 ((2R,3R,4S)-2-(6-amino-8-(hex-1-ynyl)-9H-purin-9-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells at 10 uM | ACS Med Chem Lett 9: 516-520 (2010) Article DOI: 10.1021/ml1001823 BindingDB Entry DOI: 10.7270/Q24T6KCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363172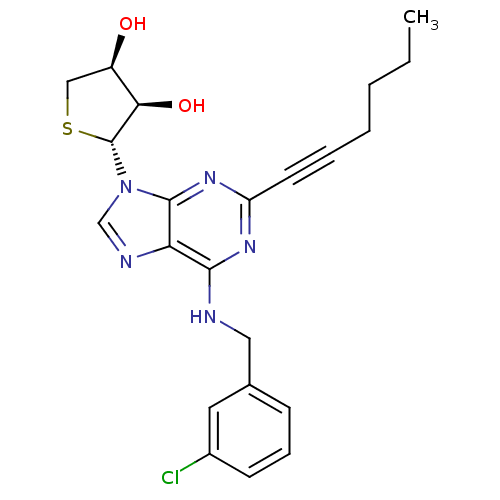 (CHEMBL1946295) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363173 (CHEMBL1946296) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363169 (CHEMBL1946299) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363174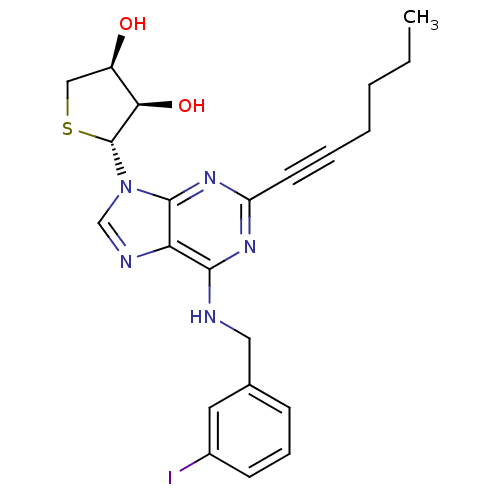 (CHEMBL1946297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363168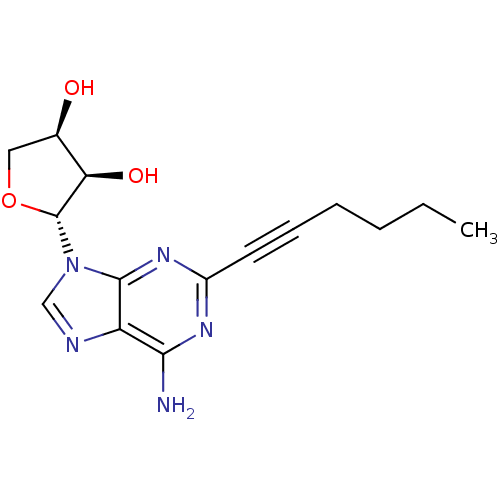 (CHEMBL1945563) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363178 (CHEMBL1946291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50339077 ((2R,3R,4S)-2-(6-amino-2-((E)-hex-1-enyl)-9H-purin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor in HEK293 cells | ACS Med Chem Lett 9: 516-520 (2010) Article DOI: 10.1021/ml1001823 BindingDB Entry DOI: 10.7270/Q24T6KCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50339077 ((2R,3R,4S)-2-(6-amino-2-((E)-hex-1-enyl)-9H-purin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363170 (CHEMBL1946301) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363168 (CHEMBL1945563) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363177 (CHEMBL1946290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363180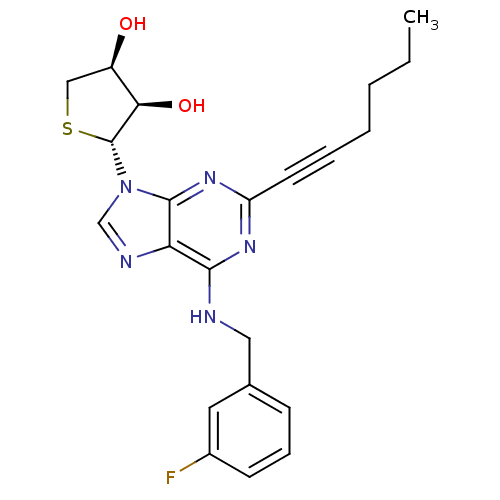 (CHEMBL1946294) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363175 (CHEMBL1946298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363175 (CHEMBL1946298) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363179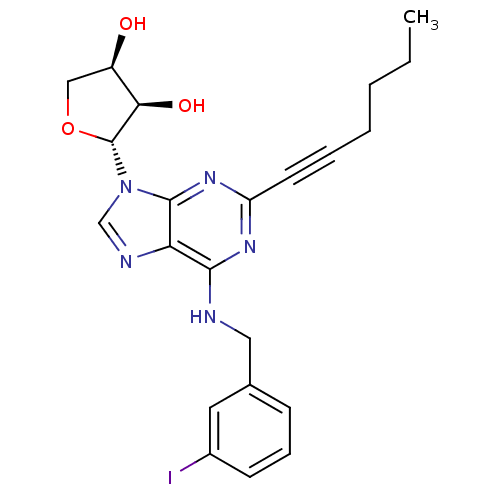 (CHEMBL1946292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50339079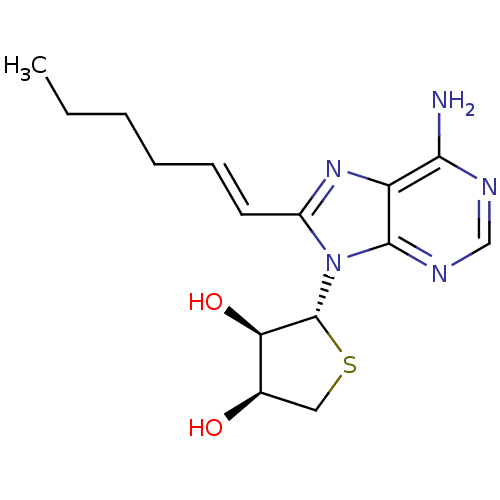 ((2R,3R,4S)-2-(6-amino-8-((E)-hex-1-enyl)-9H-purin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells at 10 uM | ACS Med Chem Lett 9: 516-520 (2010) Article DOI: 10.1021/ml1001823 BindingDB Entry DOI: 10.7270/Q24T6KCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50580162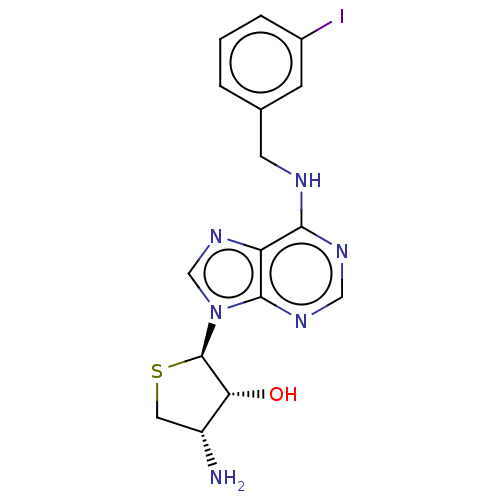 (CHEMBL5086261) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in CHO cell membrane incubated for 60 mins by gamma counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00239 BindingDB Entry DOI: 10.7270/Q2PC367C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50363169 (CHEMBL1946299) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50580161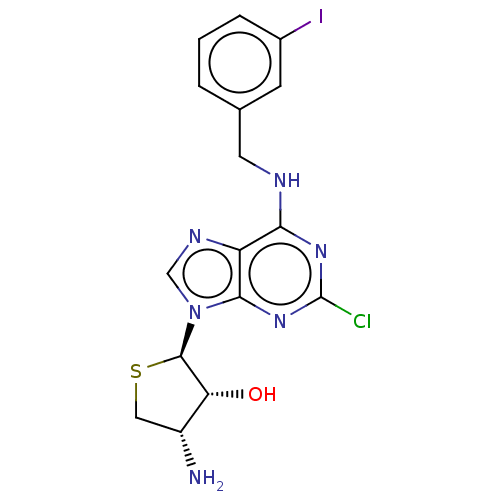 (CHEMBL5081923) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in CHO cell membrane incubated for 60 mins by gamma counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00239 BindingDB Entry DOI: 10.7270/Q2PC367C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50363170 (CHEMBL1946301) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363176 (CHEMBL1945564) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50363168 (CHEMBL1945563) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50580163 (CHEMBL5075606) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]R-PIA from recombinant human A1 adenosine receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation c... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00239 BindingDB Entry DOI: 10.7270/Q2PC367C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363171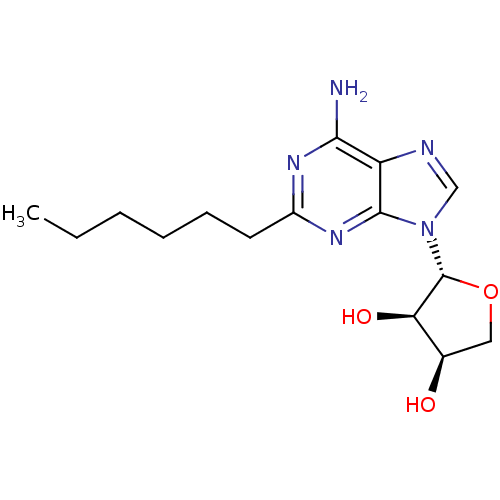 (CHEMBL1946293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50580161 (CHEMBL5081923) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]R-PIA from recombinant human A1 adenosine receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation c... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00239 BindingDB Entry DOI: 10.7270/Q2PC367C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50580162 (CHEMBL5086261) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CGS21680 from recombinant human A2A adenosine receptor expressed in HEK293 cells incubated for 60 mins by liquid scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00239 BindingDB Entry DOI: 10.7270/Q2PC367C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50580161 (CHEMBL5081923) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CGS21680 from recombinant human A2A adenosine receptor expressed in HEK293 cells incubated for 60 mins by liquid scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00239 BindingDB Entry DOI: 10.7270/Q2PC367C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50580162 (CHEMBL5086261) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]R-PIA from recombinant human A1 adenosine receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation c... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00239 BindingDB Entry DOI: 10.7270/Q2PC367C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363172 (CHEMBL1946295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363173 (CHEMBL1946296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363174 (CHEMBL1946297) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235787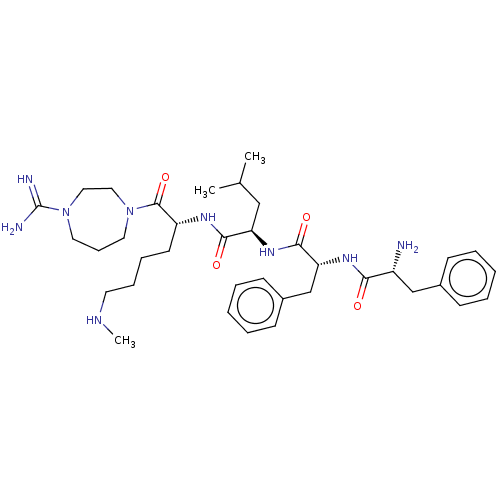 (US9359399, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0430 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235785 (CVD-0019439 | US9359399, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0480 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235793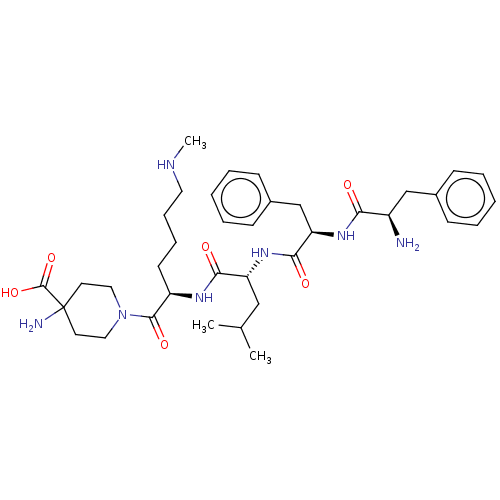 (US9359399, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0520 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235783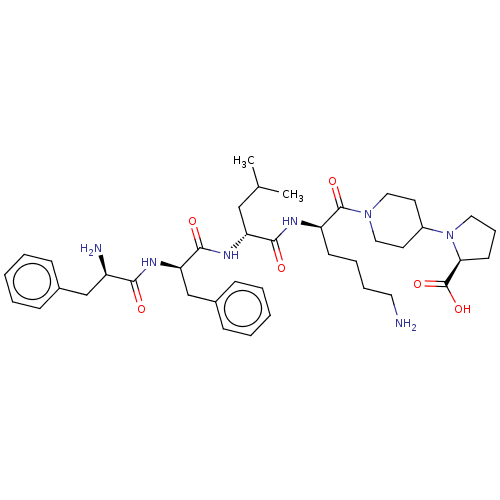 (US9359399, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0750 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235784 (US9359399, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0340 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235794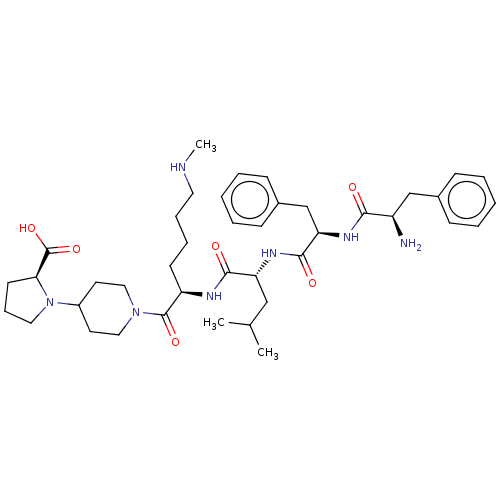 (US9359399, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0360 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235795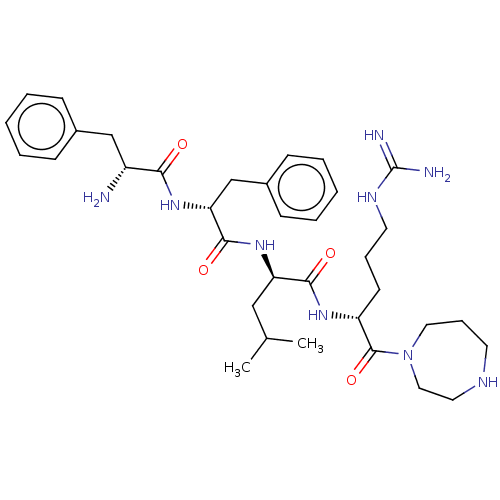 (US9359399, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0120 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235796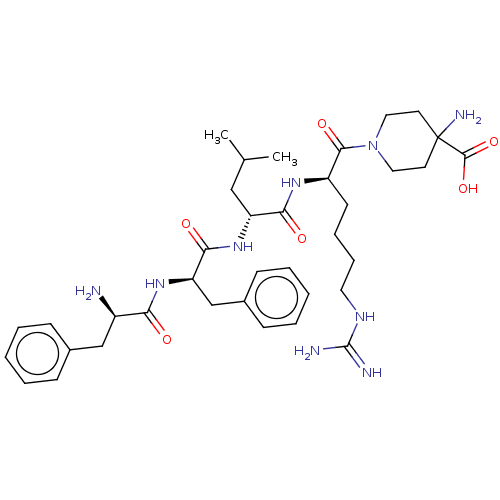 (US9359399, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0430 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235797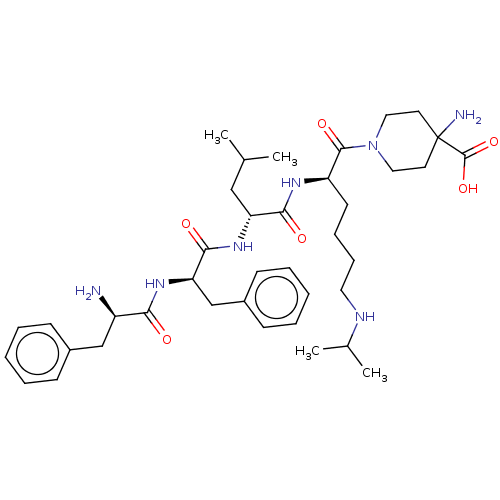 (US9359399, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0780 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM235798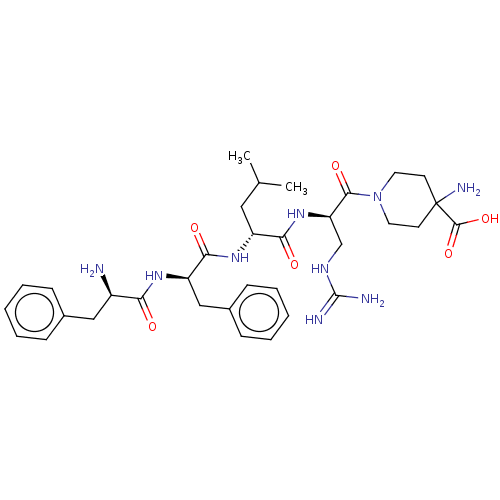 (US9359399, 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.826 | n/a | n/a | n/a | 25 |
Cara Therapeutics, Inc. US Patent | Assay Description Mouse R1.G1 cells (ATCC, Manassas, Va.) were grown in suspension in high glucose-DMEM (Dulbecco's Modified Eagle's Medium, Cellgro, Herndon, Va.) con... | US Patent US9359399 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 117 total ) | Next | Last >> |