

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit wild type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065797 (CHEMBL329488 | Sodium; 8-hydroxy-2-[(E)-2-(3,4,5-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description HIV integrase inhibitory potency of the compound was evaluated as IC50 on 3' processing of target DNA. | J Med Chem 41: 2846-57 (1998) Article DOI: 10.1021/jm980043e BindingDB Entry DOI: 10.7270/Q21Z43JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50174413 (2-(2-Diethylamino-ethyl)-9-hydroxy-5,11-dimethyl-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit D816V type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065793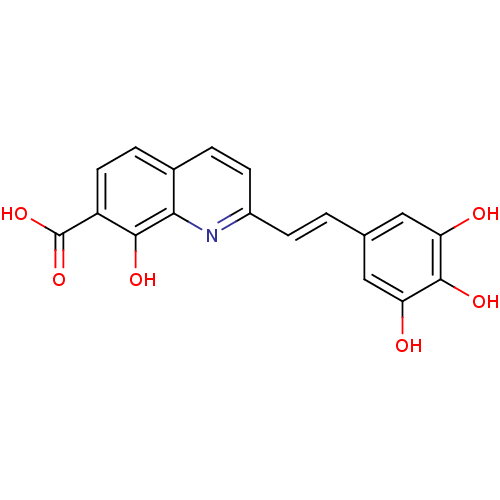 (8-Hydroxy-2-[(E)-2-(3,4,5-trihydroxy-phenyl)-vinyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against Integrase in 3'-end- processing | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065793 (8-Hydroxy-2-[(E)-2-(3,4,5-trihydroxy-phenyl)-vinyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description HIV integrase inhibitory potency of the compound was evaluated as IC50 on 3' processing of target DNA. | J Med Chem 41: 2846-57 (1998) Article DOI: 10.1021/jm980043e BindingDB Entry DOI: 10.7270/Q21Z43JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065793 (8-Hydroxy-2-[(E)-2-(3,4,5-trihydroxy-phenyl)-vinyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 8532 Curated by ChEMBL | Assay Description Inhibition of human immunodeficiency virus-1 (HIV-1) integrase. | J Med Chem 43: 1949-57 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q2RB759N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065793 (8-Hydroxy-2-[(E)-2-(3,4,5-trihydroxy-phenyl)-vinyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description In vitro anti-HIV integrase activity of the compound was tested against integration(strand transfer) of target plasmid. | J Med Chem 41: 2846-57 (1998) Article DOI: 10.1021/jm980043e BindingDB Entry DOI: 10.7270/Q21Z43JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50004227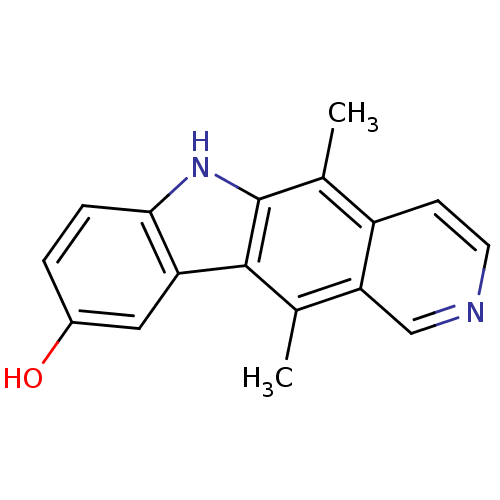 (5,11-Dimethyl-6H-pyrido[4,3-b]carbazol-9-ol | 5,11...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit wild type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50004227 (5,11-Dimethyl-6H-pyrido[4,3-b]carbazol-9-ol | 5,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory activity against Fibroblast growth factor receptor 3 | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50004235 ((NMHE)9-Hydroxy-2,5,11-trimethyl-6H-pyrido[4,3-b]c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit wild type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50004235 ((NMHE)9-Hydroxy-2,5,11-trimethyl-6H-pyrido[4,3-b]c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit D816V type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50004227 (5,11-Dimethyl-6H-pyrido[4,3-b]carbazol-9-ol | 5,11...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit D816V type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065793 (8-Hydroxy-2-[(E)-2-(3,4,5-trihydroxy-phenyl)-vinyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50174413 (2-(2-Diethylamino-ethyl)-9-hydroxy-5,11-dimethyl-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit wild type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50004235 ((NMHE)9-Hydroxy-2,5,11-trimethyl-6H-pyrido[4,3-b]c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory activity against Fibroblast growth factor receptor 3 | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065788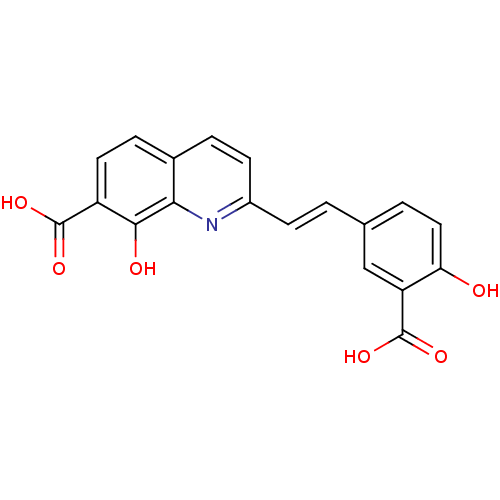 ((E)-2-(3-carboxy-4-hydroxystyryl)-8-hydroxyquinoli...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description In vitro anti-HIV integrase activity of the compound was tested against integration(strand transfer) of target plasmid. | J Med Chem 41: 2846-57 (1998) Article DOI: 10.1021/jm980043e BindingDB Entry DOI: 10.7270/Q21Z43JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50174412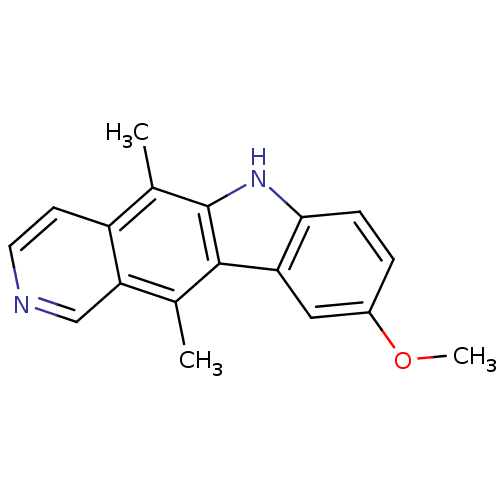 (9-Methoxy-5,11-dimethyl-6H-pyrido[4,3-b]carbazole ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit D816V type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50004227 (5,11-Dimethyl-6H-pyrido[4,3-b]carbazol-9-ol | 5,11...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory activity against Platelet-derived growth factor receptor alpha | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50004235 ((NMHE)9-Hydroxy-2,5,11-trimethyl-6H-pyrido[4,3-b]c...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory activity against Platelet-derived growth factor receptor alpha | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087419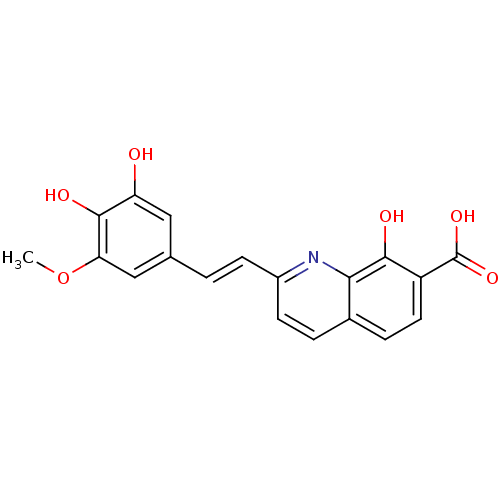 ((E)-2-(3,4-dihydroxy-5-methoxystyryl)-8-hydroxyqui...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against Integrase in 3'-end- processing | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065795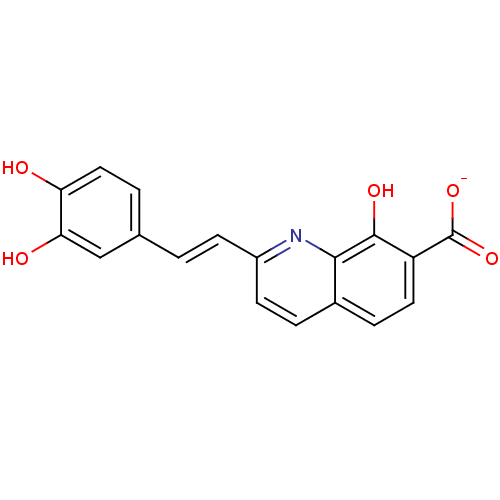 (CHEMBL100082 | Sodium; 2-[(E)-2-(3,4-dihydroxy-phe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description HIV integrase inhibitory potency of the compound was evaluated as IC50 on 3' processing of target DNA. | J Med Chem 41: 2846-57 (1998) Article DOI: 10.1021/jm980043e BindingDB Entry DOI: 10.7270/Q21Z43JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50174412 (9-Methoxy-5,11-dimethyl-6H-pyrido[4,3-b]carbazole ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit wild type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087427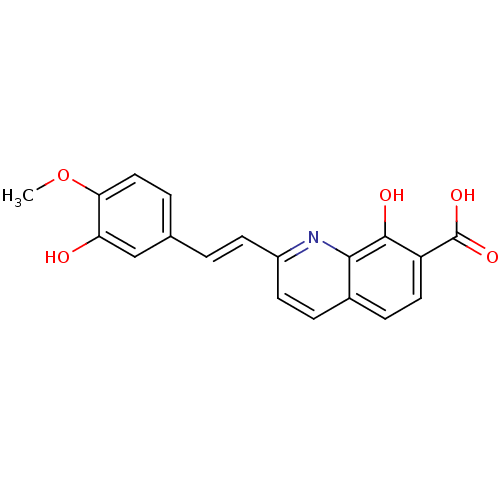 ((E)-8-hydroxy-2-(3-hydroxy-4-methoxystyryl)quinoli...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in 3'-end- processing | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065796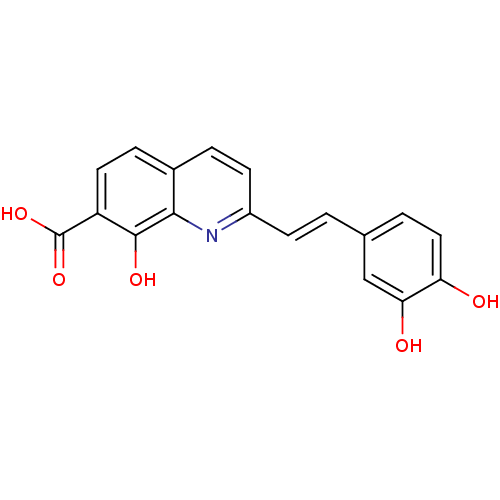 ((E)-2-(3,4-dihydroxystyryl)-8-hydroxyquinoline-7-c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065796 ((E)-2-(3,4-dihydroxystyryl)-8-hydroxyquinoline-7-c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description In vitro anti-HIV integrase activity of the compound was tested against integration(strand transfer) of target plasmid. | J Med Chem 41: 2846-57 (1998) Article DOI: 10.1021/jm980043e BindingDB Entry DOI: 10.7270/Q21Z43JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087431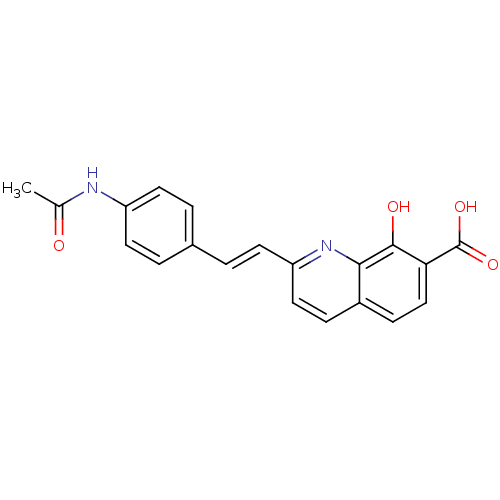 (2-[2-(4-Acetylamino-phenyl)-vinyl]-8-hydroxy-quino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087429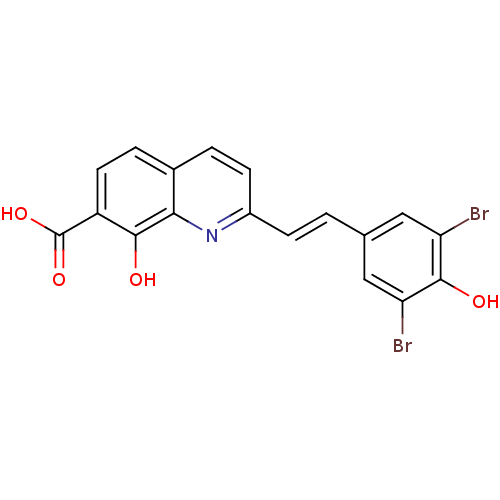 ((E)-2-(3,5-dibromo-4-hydroxystyryl)-8-hydroxyquino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50174410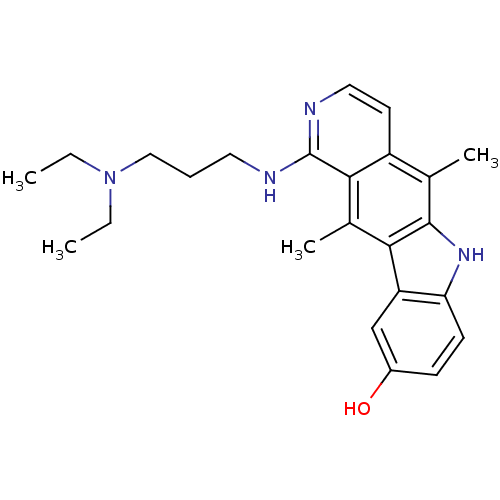 (1-(3-Diethylamino-propylamino)-5,11-dimethyl-6H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit D816V type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087432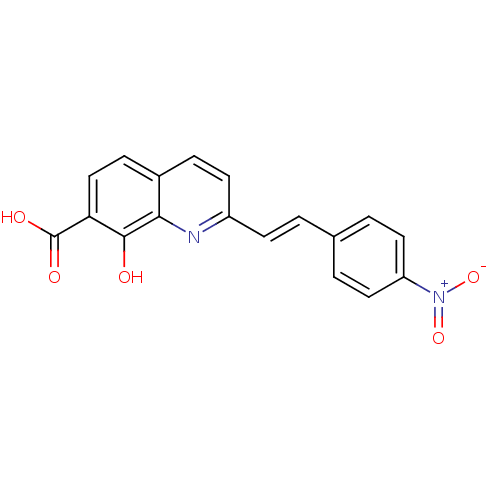 (8-Hydroxy-2-[2-(4-nitro-phenyl)-vinyl]-quinoline-7...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in 3'-end- processing | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087429 ((E)-2-(3,5-dibromo-4-hydroxystyryl)-8-hydroxyquino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in 3'-end- processing | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087431 (2-[2-(4-Acetylamino-phenyl)-vinyl]-8-hydroxy-quino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in 3'-end- processing | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50174410 (1-(3-Diethylamino-propylamino)-5,11-dimethyl-6H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit wild type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50174408 (1-(3-Diethylamino-propylamino)-5,6,11-trimethyl-6H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory activity against Fibroblast growth factor receptor 3 | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087426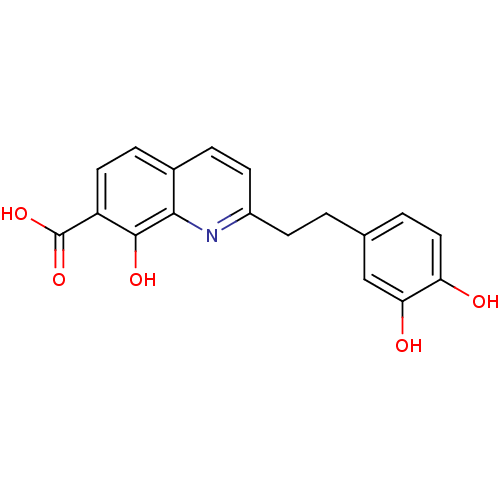 (2-(3,4-dihydroxyphenethyl)-8-hydroxyquinoline-7-ca...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087435 ((E)-8-hydroxy-2-(4-hydroxystyryl)quinoline-7-carbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in 3'-end- processing | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087435 ((E)-8-hydroxy-2-(4-hydroxystyryl)quinoline-7-carbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087419 ((E)-2-(3,4-dihydroxy-5-methoxystyryl)-8-hydroxyqui...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087432 (8-Hydroxy-2-[2-(4-nitro-phenyl)-vinyl]-quinoline-7...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50174411 (CHEMBL197610 | N,N-Diethyl-N'-(10-methyl-11H-pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit D816V type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087430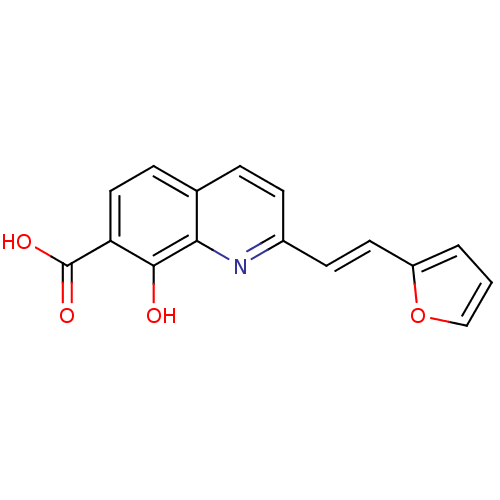 ((E)-2-(2-(furan-2-yl)vinyl)-8-hydroxyquinoline-7-c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in 3'-end- processing was tested in CEM cells | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50174408 (1-(3-Diethylamino-propylamino)-5,6,11-trimethyl-6H...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory activity against Platelet-derived growth factor receptor alpha | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50174407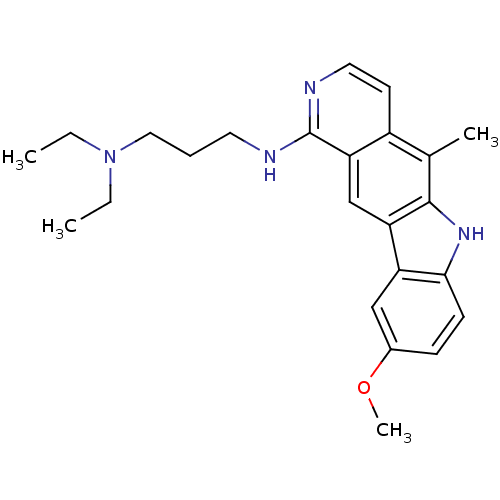 (CHEMBL279472 | N,N-Diethyl-N'-(9-methoxy-5-methyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit D816V type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087421 (8-Hydroxy-2-((E)-styryl)-quinoline-7-carboxylic ac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Rous sarcoma virus (strain Prague C) (RSV-PrC)) | BDBM50065793 (8-Hydroxy-2-[(E)-2-(3,4,5-trihydroxy-phenyl)-vinyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 8532 Curated by ChEMBL | Assay Description Inhibition of rous sarcoma virus (RSV) Integrase. | J Med Chem 43: 1949-57 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q2RB759N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065783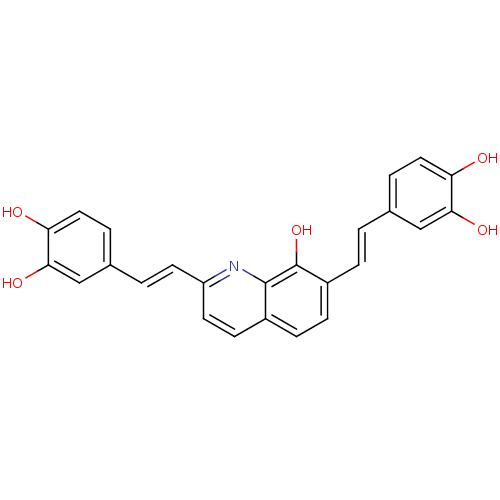 (5-(2-{7-[2-(3,4-Dihydroxy-phenyl)-vinyl]-8-hydroxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description HIV integrase inhibitory potency of the compound was evaluated as IC50 on 3' processing of target DNA. | J Med Chem 41: 2846-57 (1998) Article DOI: 10.1021/jm980043e BindingDB Entry DOI: 10.7270/Q21Z43JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087420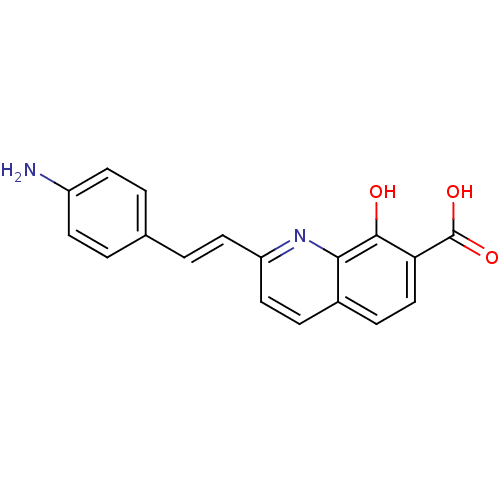 ((E)-2-(4-aminostyryl)-8-hydroxyquinoline-7-carboxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase in strand transfer(integration) | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50174411 (CHEMBL197610 | N,N-Diethyl-N'-(10-methyl-11H-pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR-8113 Curated by ChEMBL | Assay Description Inhibitory concentration against c-Kit wild type expressed in recombinant baculovirus | J Med Chem 48: 6194-201 (2005) Article DOI: 10.1021/jm050231m BindingDB Entry DOI: 10.7270/Q2B27TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50087426 (2-(3,4-dihydroxyphenethyl)-8-hydroxyquinoline-7-ca...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description Inhibitory activity against Integrase in 3'-end- processing | J Med Chem 43: 1533-40 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Rous sarcoma virus (strain Prague C) (RSV-PrC)) | BDBM50065796 ((E)-2-(3,4-dihydroxystyryl)-8-hydroxyquinoline-7-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 8532 Curated by ChEMBL | Assay Description Inhibition of rous sarcoma virus (RSV) Integrase. | J Med Chem 43: 1949-57 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q2RB759N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50065796 ((E)-2-(3,4-dihydroxystyryl)-8-hydroxyquinoline-7-c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris-Sud Curated by ChEMBL | Assay Description HIV integrase inhibitory potency of the compound was evaluated as IC50 on 3' processing of target DNA. | J Med Chem 41: 2846-57 (1998) Article DOI: 10.1021/jm980043e BindingDB Entry DOI: 10.7270/Q21Z43JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 133 total ) | Next | Last >> |