

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of Human kideny renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of marmoset plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of marmoset plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of porcine plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of Human plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022586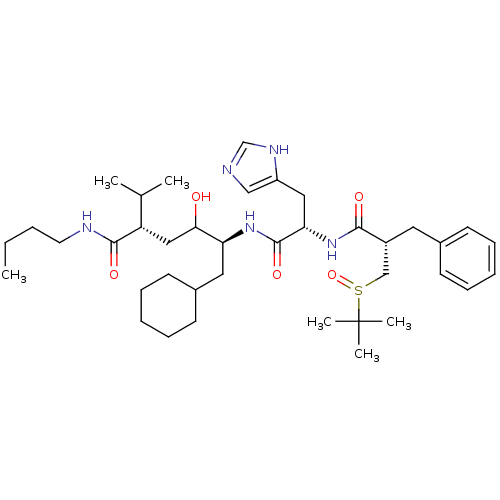 (5-[2-[2-Benzyl-3-(2-methyl-propane-2-sulfinyl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022587 (5-[2-(2-tert-Butylsulfanylmethyl-3-phenyl-propiony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022588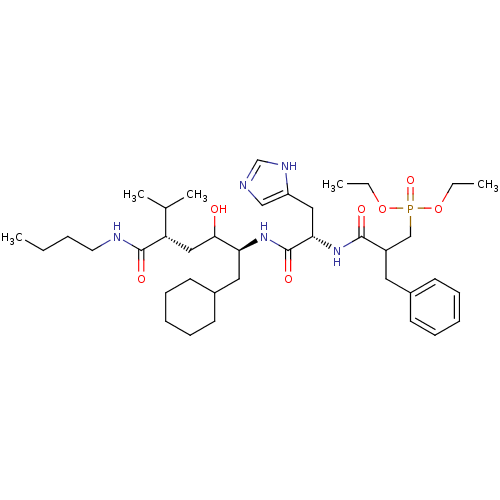 (CHEMBL291787 | {2-[1-(4-Butylcarbamoyl-1-cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022584 (2-Benzyl-5,5-dimethyl-4-oxo-hexanoic acid [1-(4-bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022590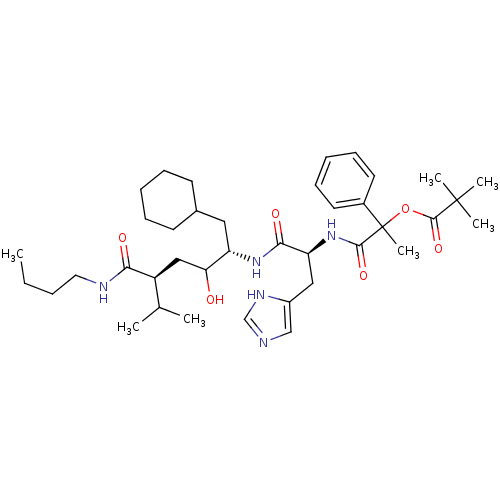 (2,2-Dimethyl-propionic acid 1-[1-(4-butylcarbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022859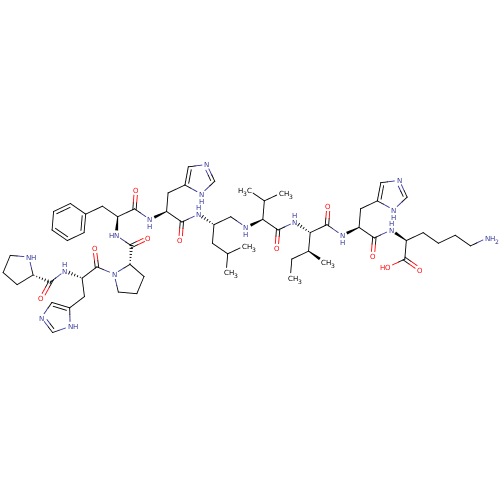 (CHEMBL407670 | Pro-His-Pro-Phe-His-Leu[CH2NH]Val-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022589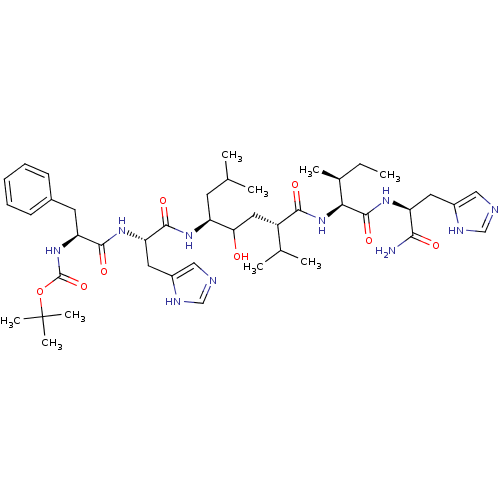 (CHEMBL435178 | {1-[1-(4-{1-[1-Carbamoyl-2-(3H-imid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022585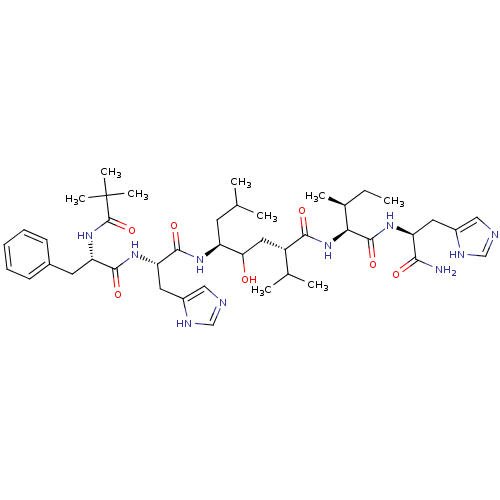 (5-[2-[2-(2,2-Dimethyl-propionylamino)-3-phenyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022581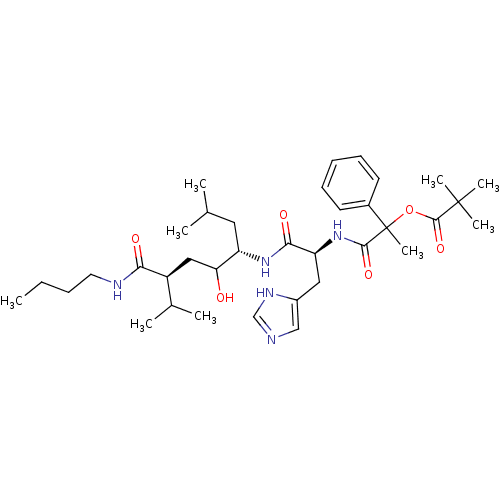 (2,2-Dimethyl-propionic acid 1-[1-(4-butylcarbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022591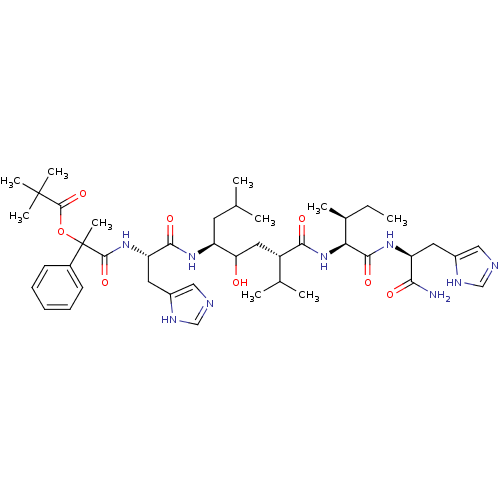 (2,2-Dimethyl-propionic acid 1-[1-(4-{1-[1-carbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50009714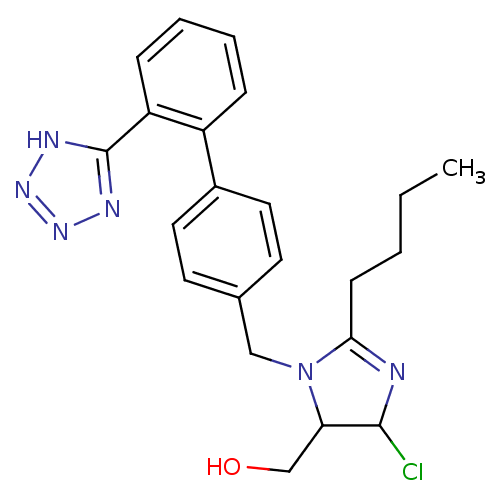 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010146 (CHEMBL322683 | {2-Butyl-5-chloro-1-[6-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against Angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of rat plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010144 (CHEMBL322071 | {2-Butyl-5-chloro-3-[6-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010154 (6-(2-Butyl-5-chloro-4-hydroxymethyl-imidazol-1-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010150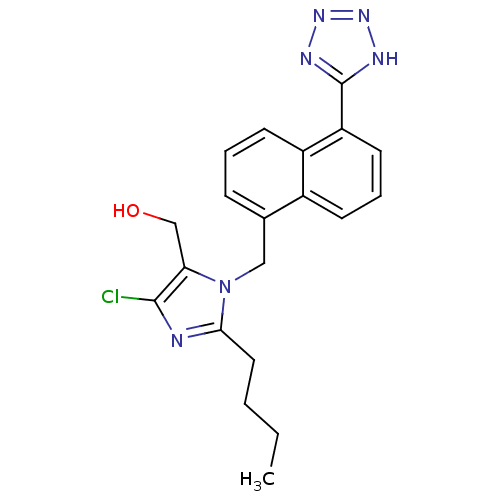 (CHEMBL107862 | {2-Butyl-5-chloro-3-[5-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010145 (CHEMBL105525 | {2-Butyl-5-chloro-1-[6-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010147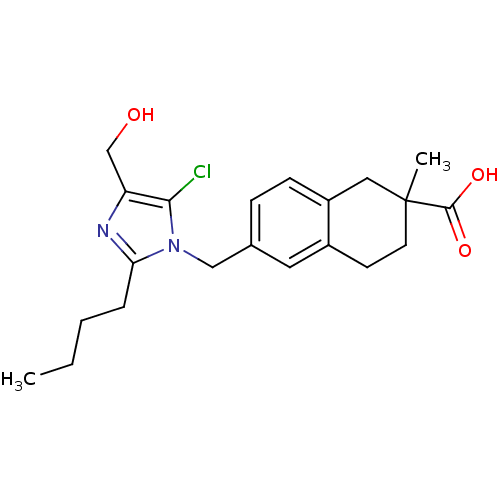 (6-(2-Butyl-5-chloro-4-hydroxymethyl-imidazol-1-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of bovine cathepsin D | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010155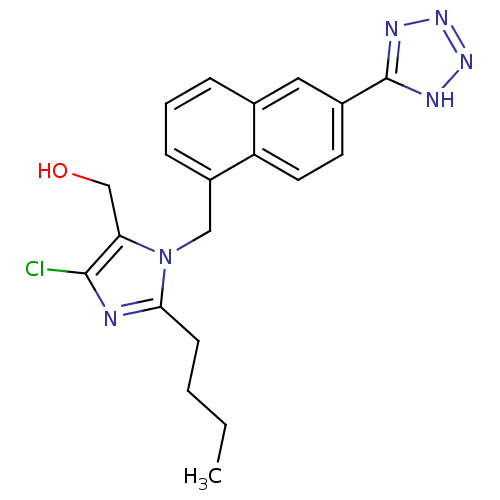 (CHEMBL320951 | {2-Butyl-5-chloro-3-[6-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010151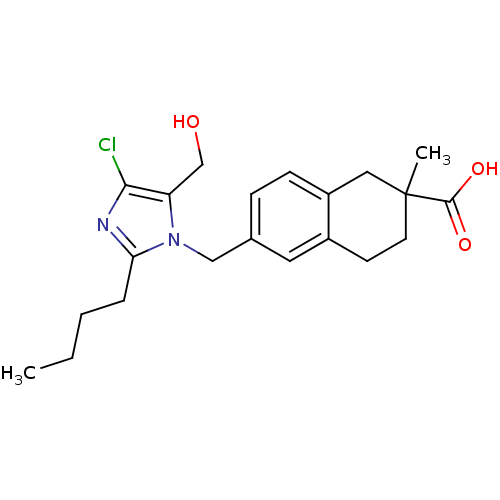 (6-(2-Butyl-4-chloro-5-hydroxymethyl-imidazol-1-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010148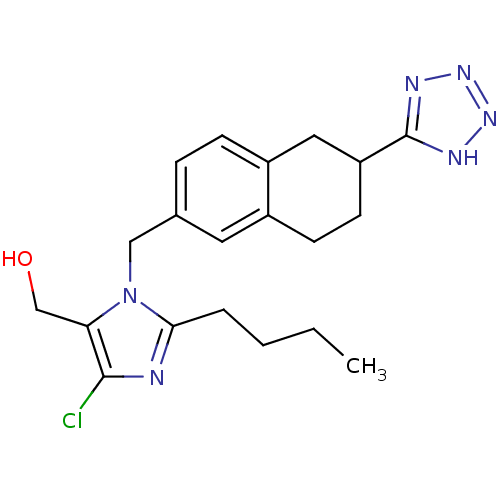 (CHEMBL106934 | {2-Butyl-5-chloro-3-[6-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of dog plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010152 (6-(2-Butyl-4-chloro-5-hydroxymethyl-imidazol-1-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010153 (CHEMBL105724 | {2-Butyl-5-chloro-3-[7-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against Angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50010149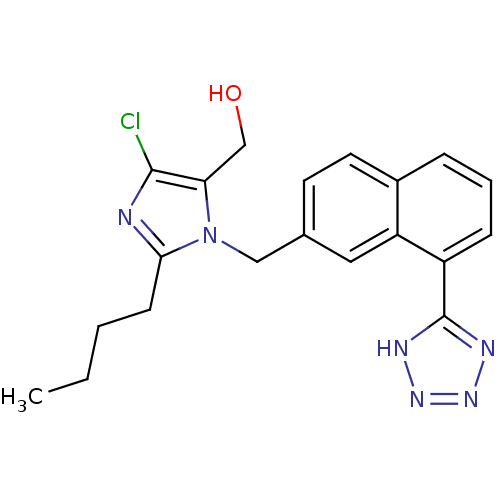 (CHEMBL323179 | {2-Butyl-5-chloro-3-[8-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Geigy Limited Curated by ChEMBL | Assay Description Binding affinity against angiotensin II receptor in rat smooth muscle cell preparations | J Med Chem 34: 3105-14 (1991) BindingDB Entry DOI: 10.7270/Q27S7MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||