 Found 214 hits with Last Name = 'bachman' and Initial = 'e'
Found 214 hits with Last Name = 'bachman' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566910
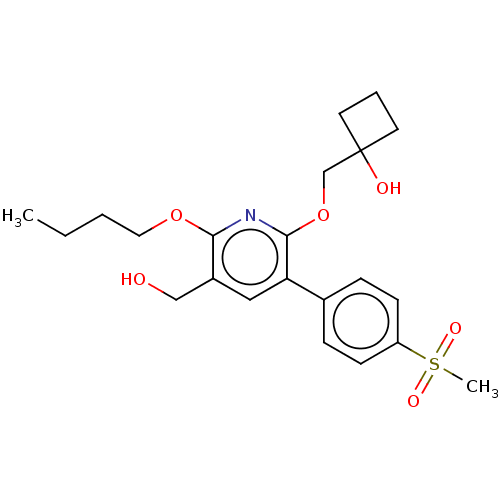
(CHEMBL4870569)Show SMILES CCCCOc1nc(OCC2(O)CCC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566901
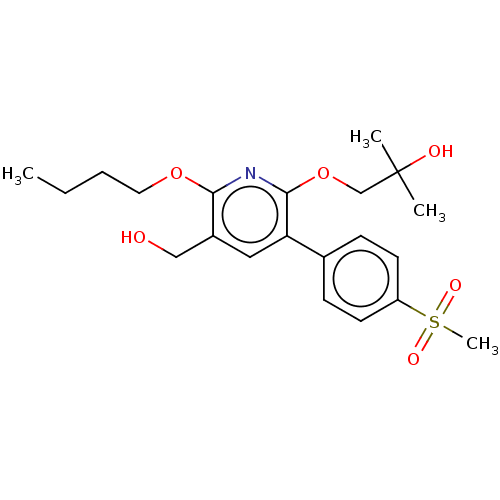
(CHEMBL4865464)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566912
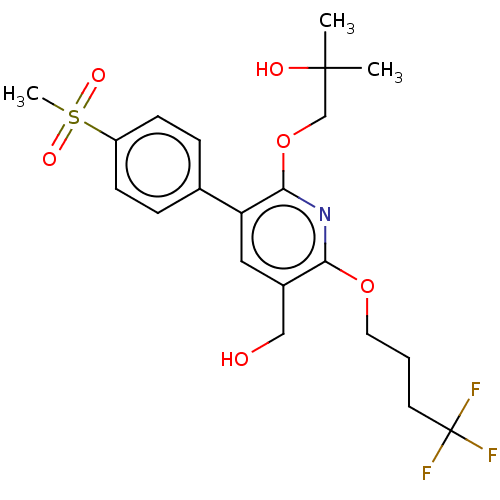
(CHEMBL4859571)Show SMILES CC(C)(O)COc1nc(OCCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566913

(CHEMBL4857474)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cn1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566911
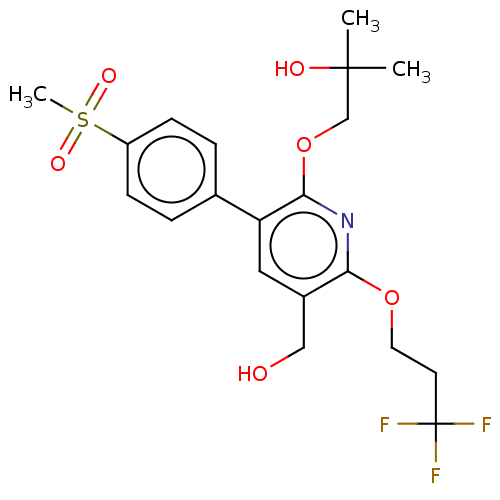
(CHEMBL4868446)Show SMILES CC(C)(O)COc1nc(OCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566900
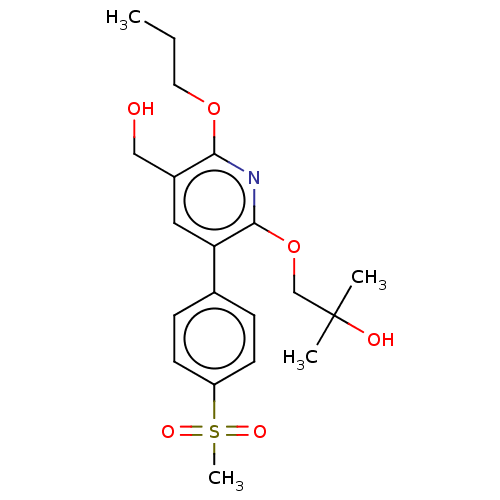
(CHEMBL4862802)Show SMILES CCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
DNA ligase 1
(Homo sapiens (Human)) | BDBM22826

(2,3-dioxo-2,3-dihydro-1H-indole-7-carboxylic acid ...)Show InChI InChI=1S/C9H5NO4/c11-7-4-2-1-3-5(9(13)14)6(4)10-8(7)12/h1-3H,(H,13,14)(H,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DNA ligase 1 using nicked DNA substrate by kinetic assay |
J Med Chem 51: 4553-62 (2008)
Article DOI: 10.1021/jm8001668
BindingDB Entry DOI: 10.7270/Q2639PJC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449372
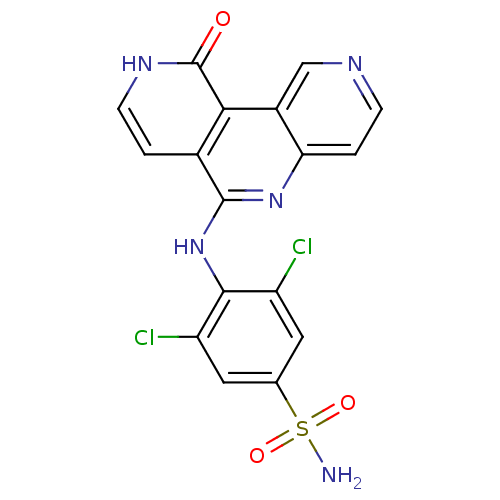
(CHEMBL3126350)Show SMILES NS(=O)(=O)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C17H11Cl2N5O3S/c18-11-5-8(28(20,26)27)6-12(19)15(11)24-16-9-1-4-22-17(25)14(9)10-7-21-3-2-13(10)23-16/h1-7H,(H,22,25)(H,23,24)(H2,20,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449373

(CHEMBL3126349)Show SMILES OC(C(F)F)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C19H12Cl2F2N4O2/c20-11-5-8(16(28)17(22)23)6-12(21)15(11)27-18-9-1-4-25-19(29)14(9)10-7-24-3-2-13(10)26-18/h1-7,16-17,28H,(H,25,29)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449375
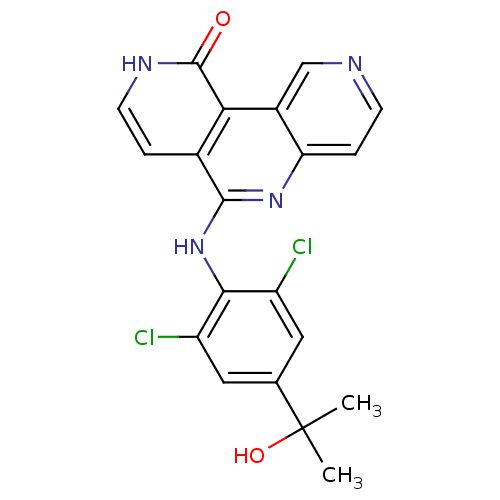
(CHEMBL3126347)Show SMILES CC(C)(O)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C20H16Cl2N4O2/c1-20(2,28)10-7-13(21)17(14(22)8-10)26-18-11-3-6-24-19(27)16(11)12-9-23-5-4-15(12)25-18/h3-9,28H,1-2H3,(H,24,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449376

(CHEMBL3126346)Show SMILES CC(O)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C19H14Cl2N4O2/c1-9(26)10-6-13(20)17(14(21)7-10)25-18-11-2-5-23-19(27)16(11)12-8-22-4-3-15(12)24-18/h2-9,26H,1H3,(H,23,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449381

(CHEMBL3126341)Show SMILES Fc1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C17H9Cl2FN4O/c18-11-5-8(20)6-12(19)15(11)24-16-9-1-4-22-17(25)14(9)10-7-21-3-2-13(10)23-16/h1-7H,(H,22,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449383

(CHEMBL3126352)Show SMILES Fc1cc(F)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C17H9ClF2N4O/c18-11-5-8(19)6-12(20)15(11)24-16-9-1-4-22-17(25)14(9)10-7-21-3-2-13(10)23-16/h1-7H,(H,22,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449378
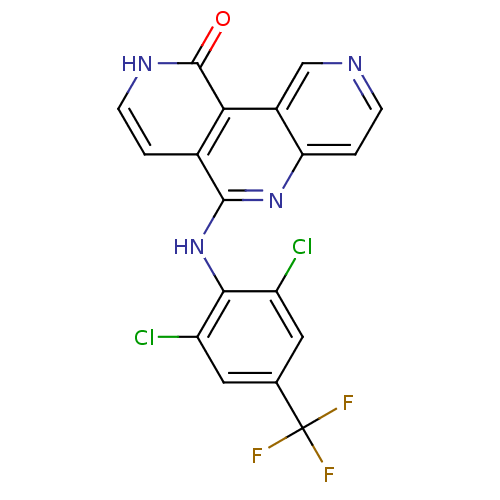
(CHEMBL3126344)Show SMILES FC(F)(F)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C18H9Cl2F3N4O/c19-11-5-8(18(21,22)23)6-12(20)15(11)27-16-9-1-4-25-17(28)14(9)10-7-24-3-2-13(10)26-16/h1-7H,(H,25,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50331809
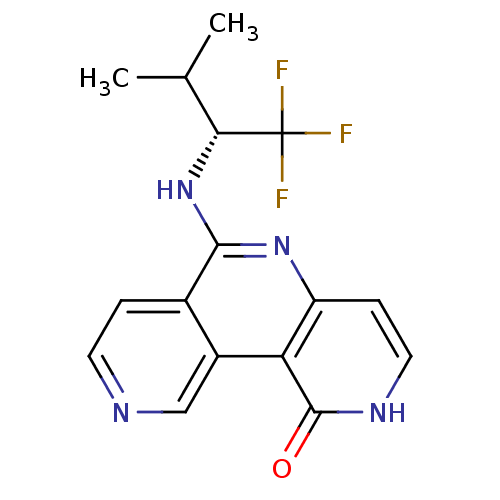
((R)-6-(1,1,1-trifluoro-3-methylbutan-2-ylamino)pyr...)Show SMILES CC(C)[C@@H](Nc1nc2cc[nH]c(=O)c2c2cnccc12)C(F)(F)F |r| Show InChI InChI=1S/C16H15F3N4O/c1-8(2)13(16(17,18)19)23-14-9-3-5-20-7-10(9)12-11(22-14)4-6-21-15(12)24/h3-8,13H,1-2H3,(H,21,24)(H,22,23)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449374

(CHEMBL3126348)Show SMILES Clc1cc(cc(Cl)c1Nc1nc2ccncc2c2c1cc[nH]c2=O)-c1cc[nH]n1 Show InChI InChI=1S/C20H12Cl2N6O/c21-13-7-10(15-3-6-25-28-15)8-14(22)18(13)27-19-11-1-5-24-20(29)17(11)12-9-23-4-2-16(12)26-19/h1-9H,(H,24,29)(H,25,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50331808
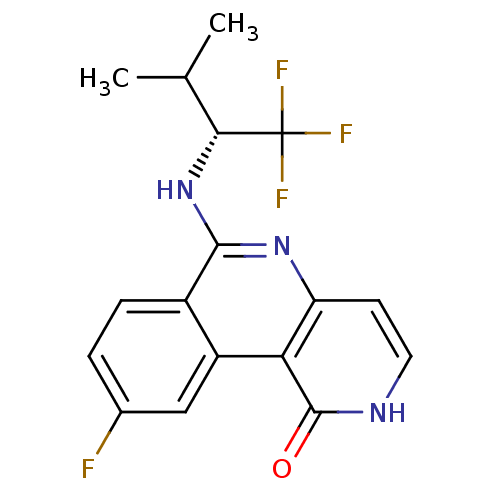
((R)-9-fluoro-6-(1,1,1-trifluoro-3-methylbutan-2-yl...)Show SMILES CC(C)[C@@H](Nc1nc2cc[nH]c(=O)c2c2cc(F)ccc12)C(F)(F)F |r| Show InChI InChI=1S/C17H15F4N3O/c1-8(2)14(17(19,20)21)24-15-10-4-3-9(18)7-11(10)13-12(23-15)5-6-22-16(13)25/h3-8,14H,1-2H3,(H,22,25)(H,23,24)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in cell-free system |
Bioorg Med Chem Lett 20: 7421-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.031
BindingDB Entry DOI: 10.7270/Q2SQ90MG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50331809

((R)-6-(1,1,1-trifluoro-3-methylbutan-2-ylamino)pyr...)Show SMILES CC(C)[C@@H](Nc1nc2cc[nH]c(=O)c2c2cnccc12)C(F)(F)F |r| Show InChI InChI=1S/C16H15F3N4O/c1-8(2)13(16(17,18)19)23-14-9-3-5-20-7-10(9)12-11(22-14)4-6-21-15(12)24/h3-8,13H,1-2H3,(H,21,24)(H,22,23)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in cell-free system |
Bioorg Med Chem Lett 20: 7421-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.031
BindingDB Entry DOI: 10.7270/Q2SQ90MG |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50566911

(CHEMBL4868446)Show SMILES CC(C)(O)COc1nc(OCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50566912

(CHEMBL4859571)Show SMILES CC(C)(O)COc1nc(OCCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449380
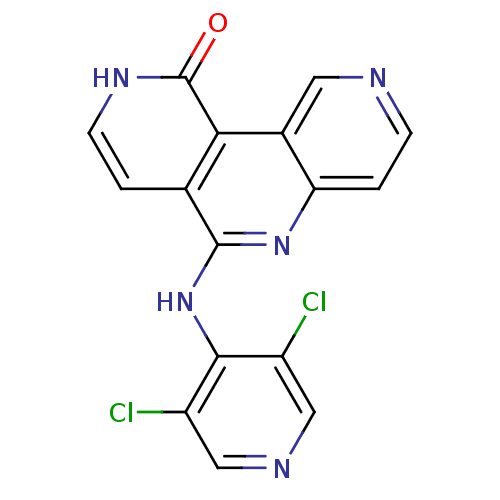
(CHEMBL3126342)Show InChI InChI=1S/C16H9Cl2N5O/c17-10-6-20-7-11(18)14(10)23-15-8-1-4-21-16(24)13(8)9-5-19-3-2-12(9)22-15/h1-7H,(H,21,24)(H,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449379

(CHEMBL3126343)Show SMILES FC(F)(F)Oc1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C18H9Cl2F3N4O2/c19-11-5-8(29-18(21,22)23)6-12(20)15(11)27-16-9-1-4-25-17(28)14(9)10-7-24-3-2-13(10)26-16/h1-7H,(H,25,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50331824
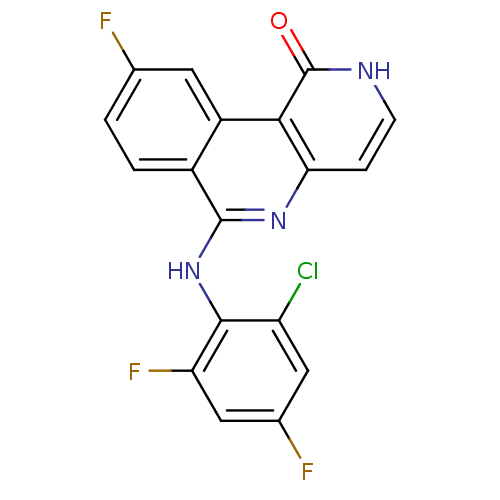
(6-(2-chloro-4,6-difluorophenylamino)-9-fluorobenzo...)Show SMILES Fc1ccc2c(Nc3c(F)cc(F)cc3Cl)nc3cc[nH]c(=O)c3c2c1 Show InChI InChI=1S/C18H9ClF3N3O/c19-12-6-9(21)7-13(22)16(12)25-17-10-2-1-8(20)5-11(10)15-14(24-17)3-4-23-18(15)26/h1-7H,(H,23,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in cell-free system |
Bioorg Med Chem Lett 20: 7421-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.031
BindingDB Entry DOI: 10.7270/Q2SQ90MG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449382
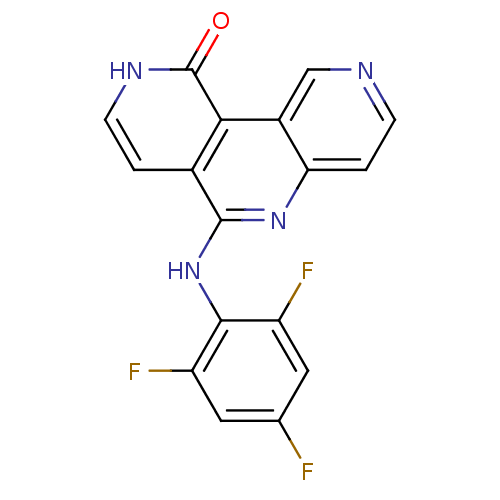
(CHEMBL3126340)Show SMILES Fc1cc(F)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(F)c1 Show InChI InChI=1S/C17H9F3N4O/c18-8-5-11(19)15(12(20)6-8)24-16-9-1-4-22-17(25)14(9)10-7-21-3-2-13(10)23-16/h1-7H,(H,22,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50003620
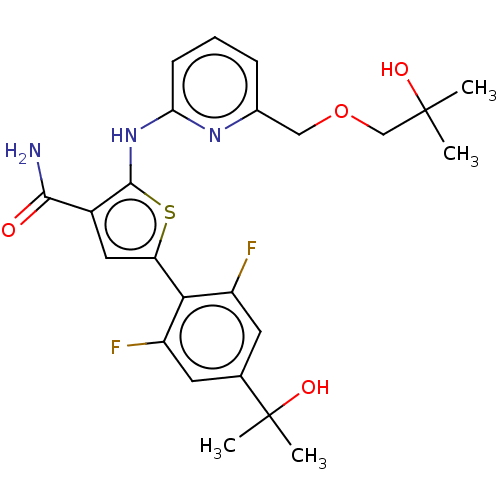
(CHEMBL3234895)Show SMILES CC(C)(O)COCc1cccc(Nc2sc(cc2C(N)=O)-c2c(F)cc(cc2F)C(C)(C)O)n1 Show InChI InChI=1S/C24H27F2N3O4S/c1-23(2,31)12-33-11-14-6-5-7-19(28-14)29-22-15(21(27)30)10-18(34-22)20-16(25)8-13(9-17(20)26)24(3,4)32/h5-10,31-32H,11-12H2,1-4H3,(H2,27,30)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 24: 1968-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.064
BindingDB Entry DOI: 10.7270/Q2CF9RM3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449377

(CHEMBL3126345)Show SMILES Fc1cccc(c1Nc1nc2ccncc2c2c1cc[nH]c2=O)C(F)(F)F Show InChI InChI=1S/C18H10F4N4O/c19-12-3-1-2-11(18(20,21)22)15(12)26-16-9-4-7-24-17(27)14(9)10-8-23-6-5-13(10)25-16/h1-8H,(H,24,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449384
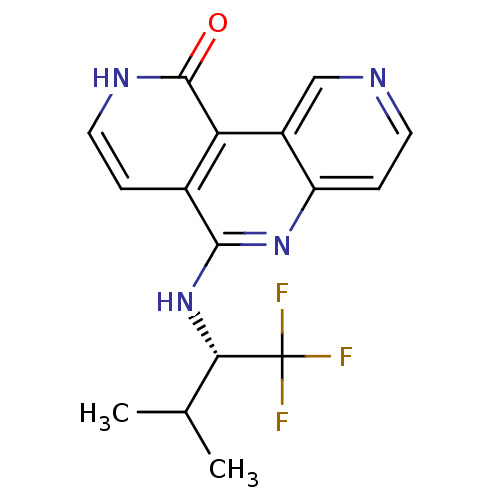
(CHEMBL3126351)Show SMILES CC(C)[C@H](Nc1nc2ccncc2c2c1cc[nH]c2=O)C(F)(F)F |r| Show InChI InChI=1S/C16H15F3N4O/c1-8(2)13(16(17,18)19)23-14-9-3-6-21-15(24)12(9)10-7-20-5-4-11(10)22-14/h3-8,13H,1-2H3,(H,21,24)(H,22,23)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449376

(CHEMBL3126346)Show SMILES CC(O)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C19H14Cl2N4O2/c1-9(26)10-6-13(20)17(14(21)7-10)25-18-11-2-5-23-19(27)16(11)12-8-22-4-3-15(12)24-18/h2-9,26H,1H3,(H,23,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) assessed as inhibition of STAT5 phosphorylation by cell-based assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50331825
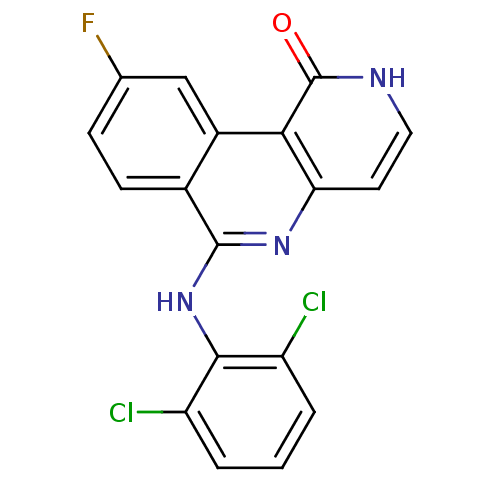
(6-(2,6-dichlorophenylamino)-9-fluorobenzo[c][1,6]n...)Show SMILES Fc1ccc2c(Nc3c(Cl)cccc3Cl)nc3cc[nH]c(=O)c3c2c1 Show InChI InChI=1S/C18H10Cl2FN3O/c19-12-2-1-3-13(20)16(12)24-17-10-5-4-9(21)8-11(10)15-14(23-17)6-7-22-18(15)25/h1-8H,(H,22,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in cell-free system |
Bioorg Med Chem Lett 20: 7421-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.031
BindingDB Entry DOI: 10.7270/Q2SQ90MG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449381

(CHEMBL3126341)Show SMILES Fc1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C17H9Cl2FN4O/c18-11-5-8(20)6-12(19)15(11)24-16-9-1-4-22-17(25)14(9)10-7-21-3-2-13(10)23-16/h1-7H,(H,22,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) assessed as inhibition of STAT5 phosphorylation by cell-based assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449383

(CHEMBL3126352)Show SMILES Fc1cc(F)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C17H9ClF2N4O/c18-11-5-8(19)6-12(20)15(11)24-16-9-1-4-22-17(25)14(9)10-7-21-3-2-13(10)23-16/h1-7H,(H,22,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) assessed as inhibition of STAT5 phosphorylation by cell-based assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50003586

(CHEMBL3234884)Show SMILES CS(=O)(=O)c1ccc(Nc2sc(cc2C(N)=O)-c2ccccc2)nc1 Show InChI InChI=1S/C17H15N3O3S2/c1-25(22,23)12-7-8-15(19-10-12)20-17-13(16(18)21)9-14(24-17)11-5-3-2-4-6-11/h2-10H,1H3,(H2,18,21)(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 24: 1968-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.064
BindingDB Entry DOI: 10.7270/Q2CF9RM3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449375

(CHEMBL3126347)Show SMILES CC(C)(O)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C20H16Cl2N4O2/c1-20(2,28)10-7-13(21)17(14(22)8-10)26-18-11-3-6-24-19(27)16(11)12-9-23-5-4-15(12)25-18/h3-9,28H,1-2H3,(H,24,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) assessed as inhibition of STAT5 phosphorylation by cell-based assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50003619

(CHEMBL3234873)Show SMILES CC(C)(O)c1ccc(cc1)-c1cc(C(N)=O)c(Nc2cccc(CN3CCOCC3)n2)s1 Show InChI InChI=1S/C24H28N4O3S/c1-24(2,30)17-8-6-16(7-9-17)20-14-19(22(25)29)23(32-20)27-21-5-3-4-18(26-21)15-28-10-12-31-13-11-28/h3-9,14,30H,10-13,15H2,1-2H3,(H2,25,29)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 24: 1968-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.064
BindingDB Entry DOI: 10.7270/Q2CF9RM3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449380

(CHEMBL3126342)Show InChI InChI=1S/C16H9Cl2N5O/c17-10-6-20-7-11(18)14(10)23-15-8-1-4-21-16(24)13(8)9-5-19-3-2-12(9)22-15/h1-7H,(H,21,24)(H,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) assessed as inhibition of STAT5 phosphorylation by cell-based assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449373

(CHEMBL3126349)Show SMILES OC(C(F)F)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C19H12Cl2F2N4O2/c20-11-5-8(16(28)17(22)23)6-12(21)15(11)27-18-9-1-4-25-19(29)14(9)10-7-24-3-2-13(10)26-18/h1-7,16-17,28H,(H,25,29)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) assessed as inhibition of STAT5 phosphorylation by cell-based assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50331817
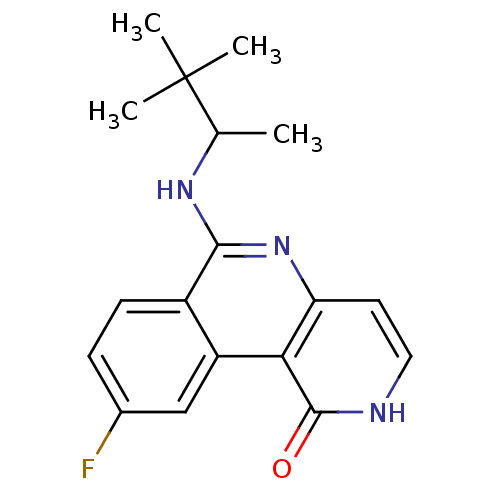
(6-(3,3-dimethylbutan-2-ylamino)-9-fluorobenzo[c][1...)Show SMILES CC(Nc1nc2cc[nH]c(=O)c2c2cc(F)ccc12)C(C)(C)C Show InChI InChI=1S/C18H20FN3O/c1-10(18(2,3)4)21-16-12-6-5-11(19)9-13(12)15-14(22-16)7-8-20-17(15)23/h5-10H,1-4H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in cell-free system |
Bioorg Med Chem Lett 20: 7421-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.031
BindingDB Entry DOI: 10.7270/Q2SQ90MG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50331829
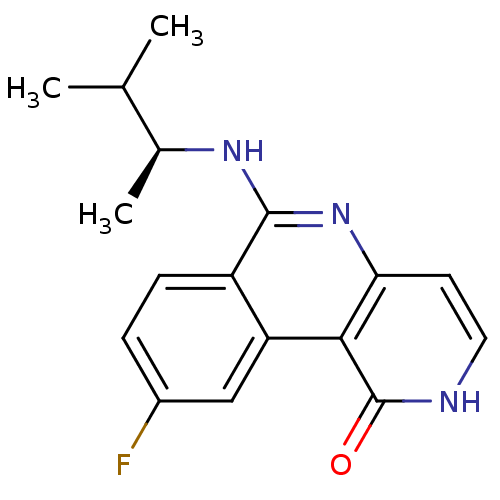
((S)-9-fluoro-6-(3-methylbutan-2-ylamino)benzo[c][1...)Show SMILES CC(C)[C@H](C)Nc1nc2cc[nH]c(=O)c2c2cc(F)ccc12 |r| Show InChI InChI=1S/C17H18FN3O/c1-9(2)10(3)20-16-12-5-4-11(18)8-13(12)15-14(21-16)6-7-19-17(15)22/h4-10H,1-3H3,(H,19,22)(H,20,21)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in cell-free system |
Bioorg Med Chem Lett 20: 7421-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.031
BindingDB Entry DOI: 10.7270/Q2SQ90MG |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in peroxidase activity using arachidonic acid as substrate preincubated for 60 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50003615
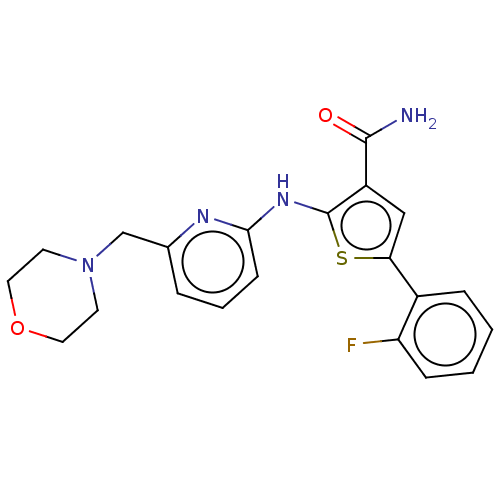
(CHEMBL3234874)Show SMILES NC(=O)c1cc(sc1Nc1cccc(CN2CCOCC2)n1)-c1ccccc1F Show InChI InChI=1S/C21H21FN4O2S/c22-17-6-2-1-5-15(17)18-12-16(20(23)27)21(29-18)25-19-7-3-4-14(24-19)13-26-8-10-28-11-9-26/h1-7,12H,8-11,13H2,(H2,23,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 24: 1968-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.064
BindingDB Entry DOI: 10.7270/Q2CF9RM3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50566900

(CHEMBL4862802)Show SMILES CCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566907
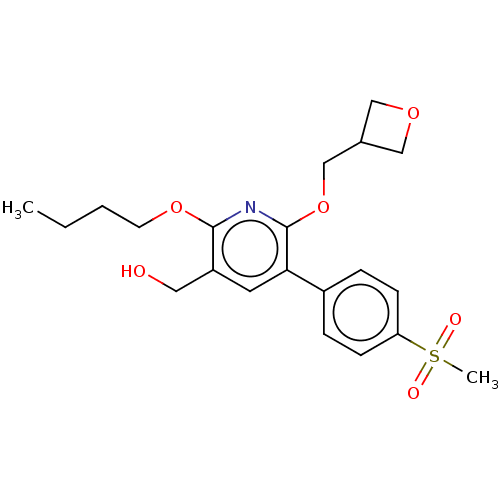
(CHEMBL4851236)Show SMILES CCCCOc1nc(OCC2COC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50003589
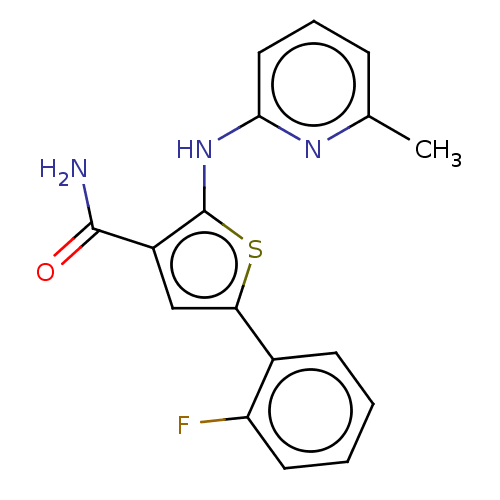
(CHEMBL3233031)Show InChI InChI=1S/C17H14FN3OS/c1-10-5-4-8-15(20-10)21-17-12(16(19)22)9-14(23-17)11-6-2-3-7-13(11)18/h2-9H,1H3,(H2,19,22)(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 24: 1968-73 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.064
BindingDB Entry DOI: 10.7270/Q2CF9RM3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566910

(CHEMBL4870569)Show SMILES CCCCOc1nc(OCC2(O)CCC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449374

(CHEMBL3126348)Show SMILES Clc1cc(cc(Cl)c1Nc1nc2ccncc2c2c1cc[nH]c2=O)-c1cc[nH]n1 Show InChI InChI=1S/C20H12Cl2N6O/c21-13-7-10(15-3-6-25-28-15)8-14(22)18(13)27-19-11-1-5-24-20(29)17(11)12-9-23-4-2-16(12)26-19/h1-9H,(H,24,29)(H,25,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) assessed as inhibition of STAT5 phosphorylation by cell-based assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50331809

((R)-6-(1,1,1-trifluoro-3-methylbutan-2-ylamino)pyr...)Show SMILES CC(C)[C@@H](Nc1nc2cc[nH]c(=O)c2c2cnccc12)C(F)(F)F |r| Show InChI InChI=1S/C16H15F3N4O/c1-8(2)13(16(17,18)19)23-14-9-3-5-20-7-10(9)12-11(22-14)4-6-21-15(12)24/h3-8,13H,1-2H3,(H,21,24)(H,22,23)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 expressed in human Du-145 cells |
Bioorg Med Chem Lett 20: 7421-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.031
BindingDB Entry DOI: 10.7270/Q2SQ90MG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data