

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50581660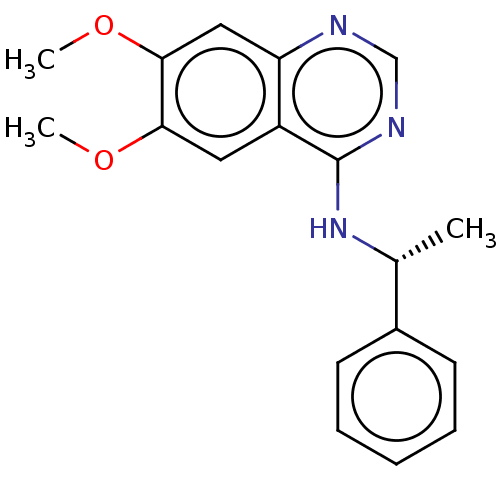 (CHEMBL1714813) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01949 BindingDB Entry DOI: 10.7270/Q2154MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519121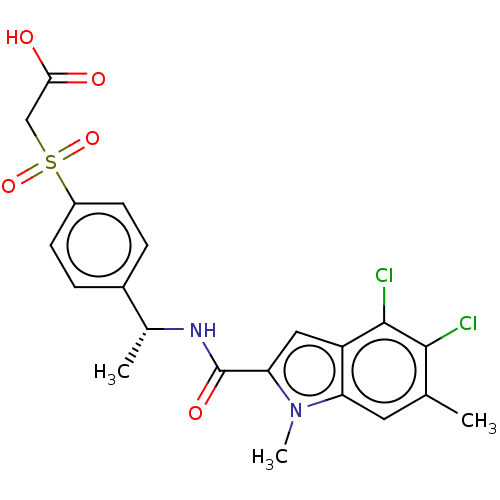 (CHEMBL4436264 | US11304929, Example 03-005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519122 (CHEMBL4467246 | US11304929, Example 03-009) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519130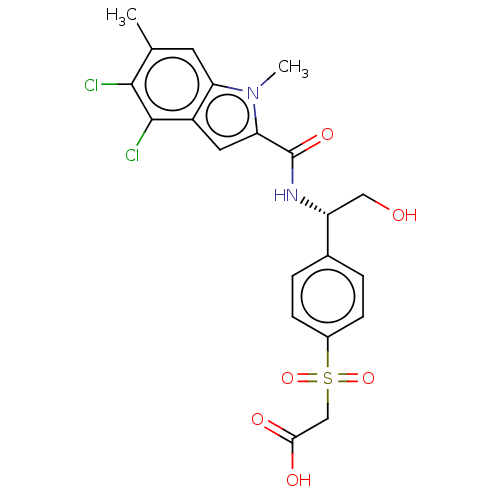 (CHEMBL4520837 | US11304929, Example 03-006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519121 (CHEMBL4436264 | US11304929, Example 03-005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519122 (CHEMBL4467246 | US11304929, Example 03-009) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50183567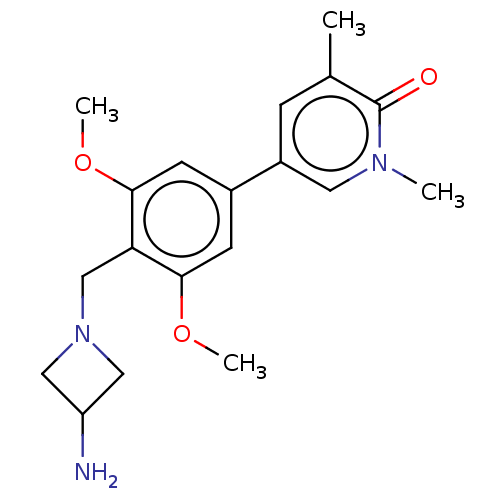 (CHEMBL3823177) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD9 isoform 1 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by Al... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519137 (CHEMBL4539932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of His6-tagged PHGDH (unknown origin) expressed in Escherichia coli BL21 assessed as effect on NADH fluorescence incubated for 60 mins usi... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50505841 (CHEMBL4532034 | US11174245, # I-018) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG Curated by ChEMBL | Assay Description Inhibition of GST-fusion tagged EGFR L858R/T790M/C797S triple mutant (unknown origin) (696 to 1022 residues) expressed in insect cells assessed as de... | J Med Chem 62: 10272-10293 (2019) Article DOI: 10.1021/acs.jmedchem.9b01169 BindingDB Entry DOI: 10.7270/Q2XD14ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50279080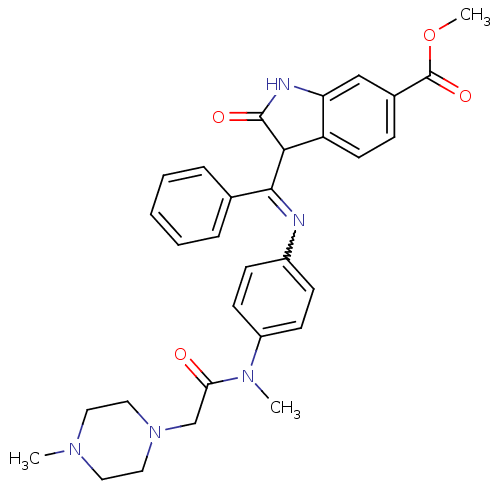 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of human VEGFR3 | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Mus musculus) | BDBM50279080 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of mouse GST-fused VEGFR2 expressed in Sf9 insect cells after 20 mins by scintillation counting | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519120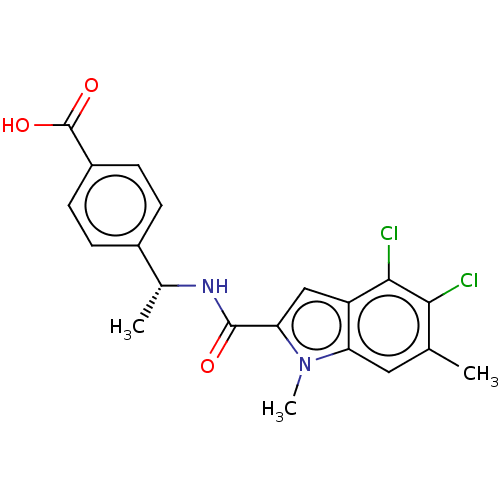 (CHEMBL4438014) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50279080 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of human Lck | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM134019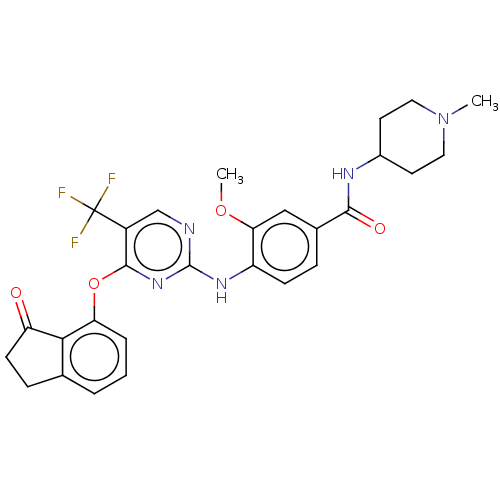 (US8846689, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PTK2 (411-689 residues) expressed in Hi-5 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01826 BindingDB Entry DOI: 10.7270/Q2MK6HH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM134019 (US8846689, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PTK2 (411-689 residues) expressed in Hi-5 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01826 BindingDB Entry DOI: 10.7270/Q2MK6HH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50548325 (CHEMBL4781145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PTK2 (411-689 residues) expressed in Hi-5 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01826 BindingDB Entry DOI: 10.7270/Q2MK6HH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50548325 (CHEMBL4781145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PTK2 (411-689 residues) expressed in Hi-5 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01826 BindingDB Entry DOI: 10.7270/Q2MK6HH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50548324 (CHEMBL4778548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PTK2 (411-689 residues) expressed in Hi-5 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01826 BindingDB Entry DOI: 10.7270/Q2MK6HH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50183448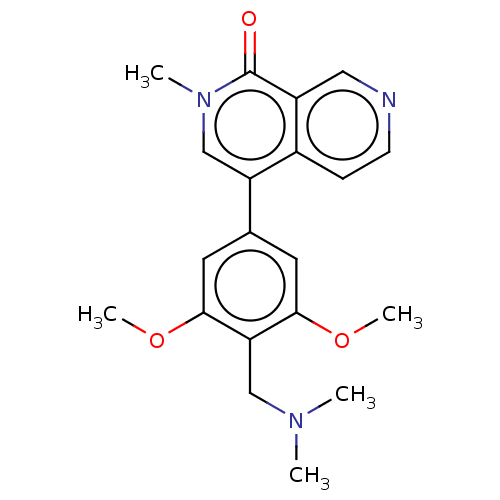 (CHEMBL3823478 | US11773085, Compound B2) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD9 isoform 1 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by Al... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50548324 (CHEMBL4778548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PTK2 (411-689 residues) expressed in Hi-5 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01826 BindingDB Entry DOI: 10.7270/Q2MK6HH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50279080 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of human GST-fused VEGFR2 expressed in Sf9 insect cells after 20 mins by scintillation counting | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50183568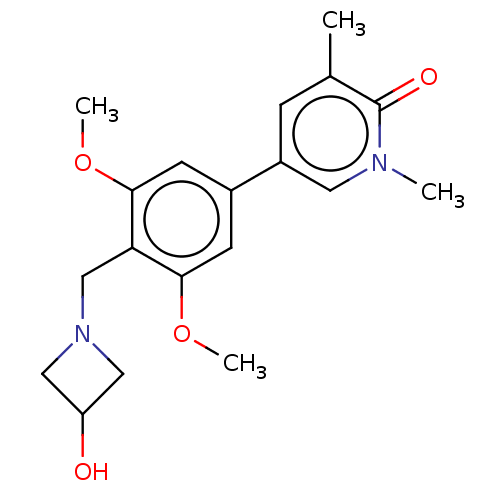 (CHEMBL3823369) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD9 isoform 1 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by Al... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519114 (CHEMBL4458393 | US11304929, Example 04-001) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50279080 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of human Flt3 | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519150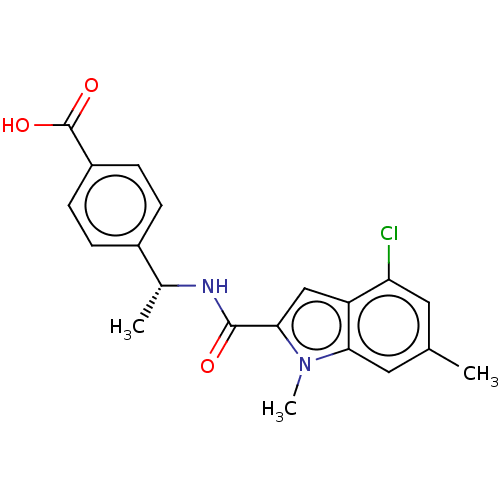 (CHEMBL4594097) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519093 (CHEMBL4522467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519131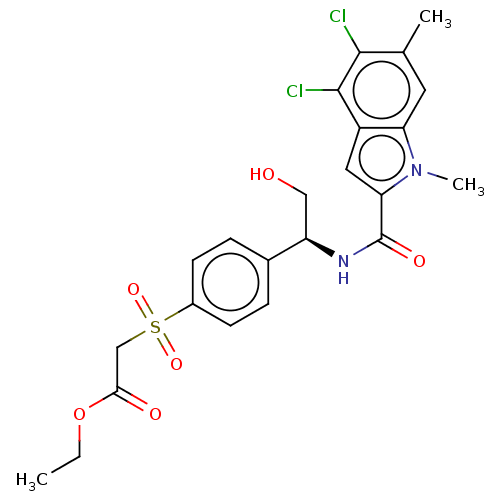 (CHEMBL4518579 | US11304929, Example 01-002) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of PHGDH in human MDA-MB-468 cells assessed as reduction in [13C]-serine incubated for 1 hr using [13C]glucose as substrate by LC-MS/MS an... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519090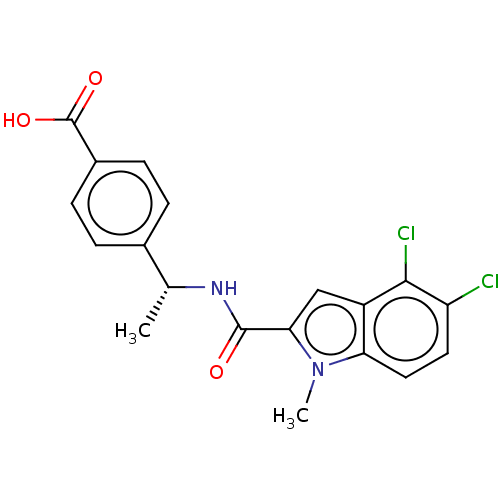 (CHEMBL4469992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519118 (CHEMBL4473052) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50183561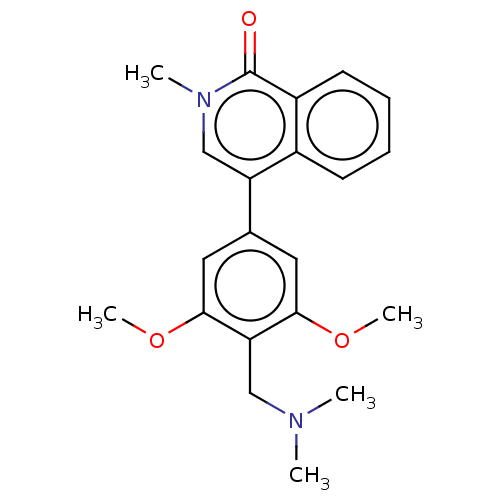 (CHEMBL3824146) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD9 isoform 1 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by Al... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50279080 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of human VEGFR1 | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 (Homo sapiens (Human)) | BDBM50581659 (CHEMBL4519023 | US20230339952, Comparative Compoun...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of SOS1-mediated proliferation of human DLD-1 cells assessed as proliferation incubated for 5 to 14 days by AlamarBlue based 3D proliferat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01949 BindingDB Entry DOI: 10.7270/Q2154MXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50279080 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of human FGFR2 | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50183453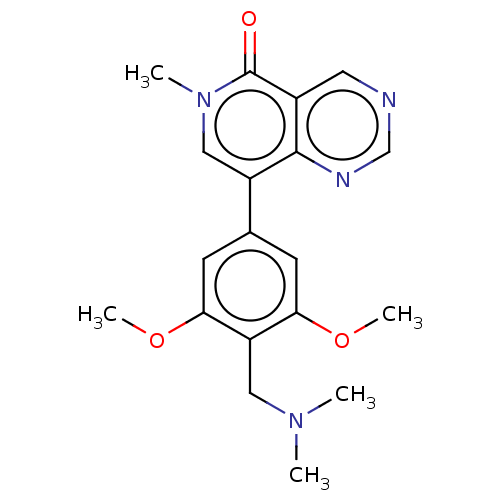 (CHEMBL3824312) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD9 isoform 1 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by Al... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50183562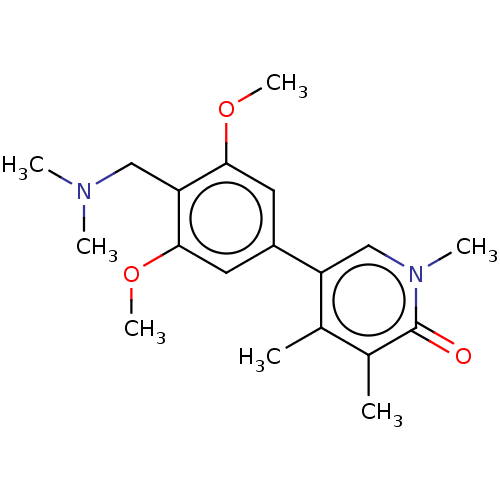 (CHEMBL3823599) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD9 isoform 1 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by Al... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 7 (Homo sapiens (Human)) | BDBM50183562 (CHEMBL3823599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD7 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by AlphaScreen ... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519117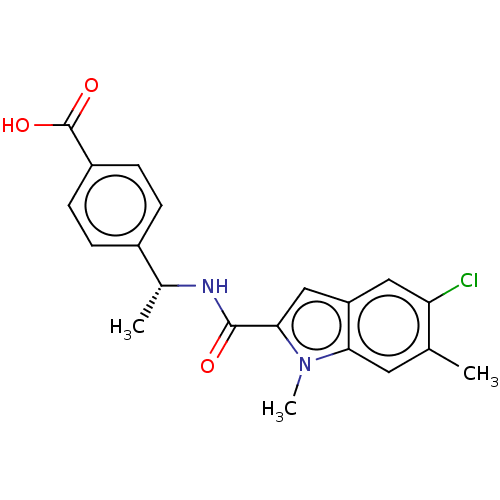 (CHEMBL4474620) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50183564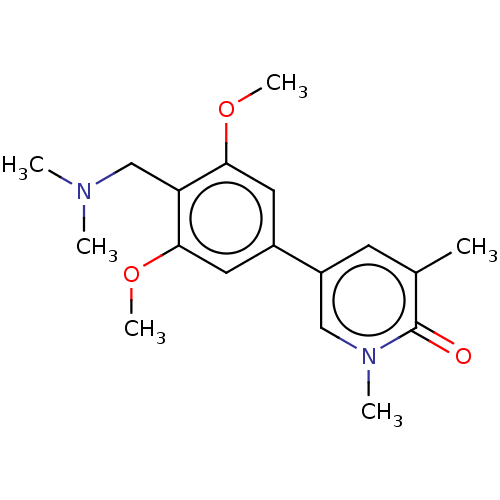 (CHEMBL3822990) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD9 isoform 1 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by Al... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50183563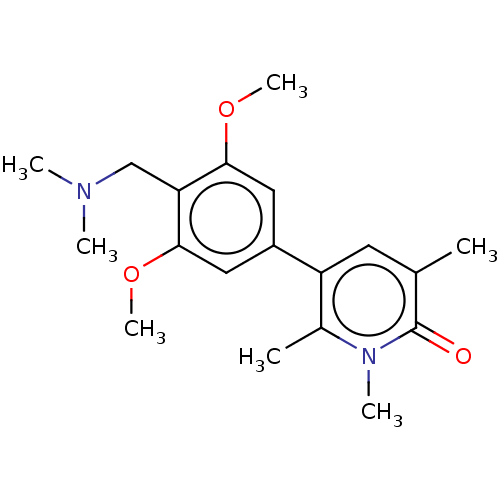 (CHEMBL3823697) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD9 isoform 1 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by Al... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519116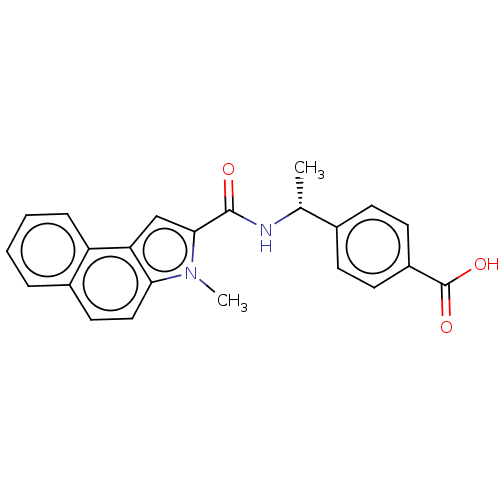 (CHEMBL4457646) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50279080 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of human PDGFRalpha | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519129 (CHEMBL4560125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50279080 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of human PDGFRbeta | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50279080 ((Z)-3-[(4-{Methyl-[2-(4-methylpiperazin-1-yl)acety...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Austria GmbH Curated by ChEMBL | Assay Description Inhibition of human FGFR1 | Cancer Res 68: 4774-82 (2008) Article DOI: 10.1158/0008-5472.CAN-07-6307 BindingDB Entry DOI: 10.7270/Q26M3715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519136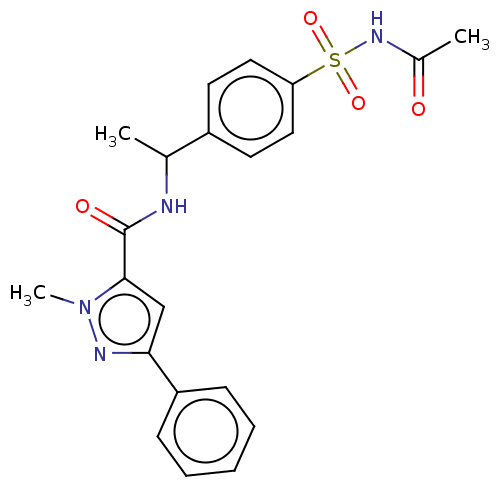 (CHEMBL4565713) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519092 (CHEMBL4437057) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50183449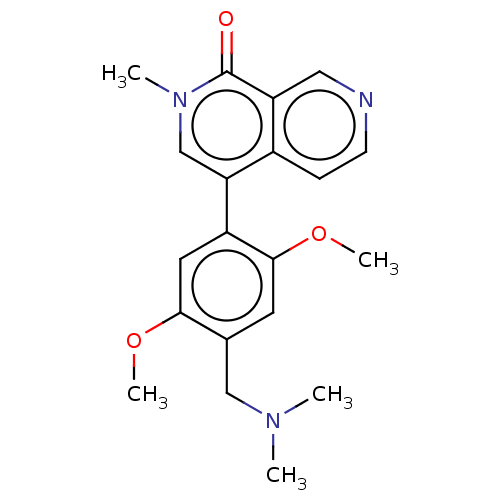 (CHEMBL3823101 | US11773085, Compound B23) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged BRD9 isoform 1 expressed in Escherichia coli using tetra-acetylated histone H4 measured after 60 mins by Al... | J Med Chem 59: 4462-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01865 BindingDB Entry DOI: 10.7270/Q27H1MJZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519119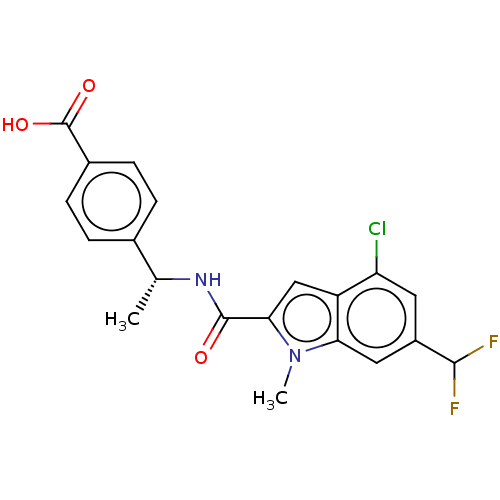 (CHEMBL4444192) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Mus musculus) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG Curated by ChEMBL | Assay Description Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... | J Med Chem 62: 10272-10293 (2019) Article DOI: 10.1021/acs.jmedchem.9b01169 BindingDB Entry DOI: 10.7270/Q2XD14ZQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50519120 (CHEMBL4438014) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human PHGDH (4 to 315 residues) assessed as effect on NADH fluorescence incubated for 2 hrs using 3-PG substrate... | J Med Chem 62: 7976-7997 (2019) Article DOI: 10.1021/acs.jmedchem.9b00718 BindingDB Entry DOI: 10.7270/Q25H7KNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 279 total ) | Next | Last >> |