

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556159 (CHEMBL4784247) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to wild type HIV-1 p66/p51 reverse transcriptase/nucleic acid/dTTP ternary complex using poly(rA)/oligo(dT) as templates in presence... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556159 (CHEMBL4784247) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to free form of wild type HIV-1 p66/p51 reverse transcriptase using poly(rA)/oligo(dT) as templates in presence of [3H]dTTP | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556159 (CHEMBL4784247) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to wild type HIV-1 p66/p51 reverse transcriptase/nucleic acid binary complex using poly(rA)/oligo(dT) as templates in presence of [3... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50422387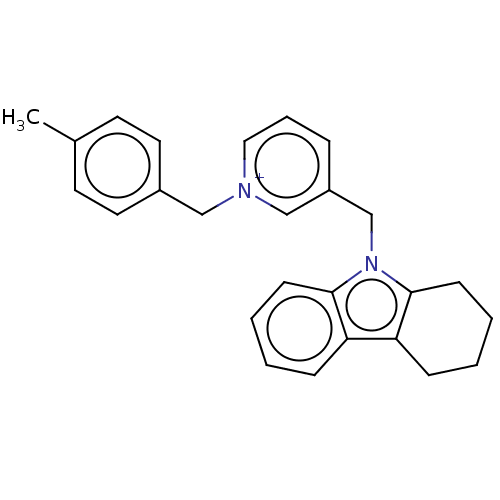 (CHEMBL4159171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BChE using varying levels of butyrylthiocholine iodide as substrate by Lineweaver-burk plot analysis | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464028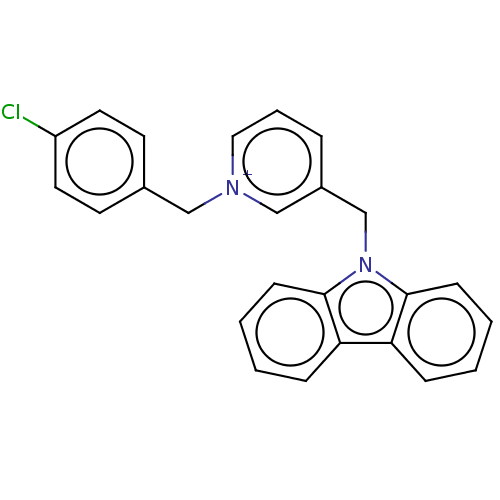 (CHEMBL4241164) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE using varying levels of butyrylthiocholine iodide as substrate preincubated for 10 mins followed by subst... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50422394 (CHEMBL3558149) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033881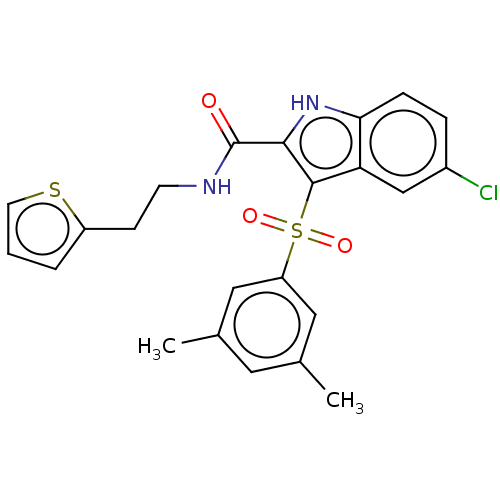 (CHEMBL3358173) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556161 (CHEMBL4759261) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RNA-dependent DNA polymerase activity of recombinant HIV-1 p66/p51 reverse transcriptase K103N mutant assessed as inhibition of [3H]dTT... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 (1 to 460 residues) expressed in baculovirus-infected insect cells using Rh-EVNLDAEFK-quencher as substrate mea... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033878 (CHEMBL3358170) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033863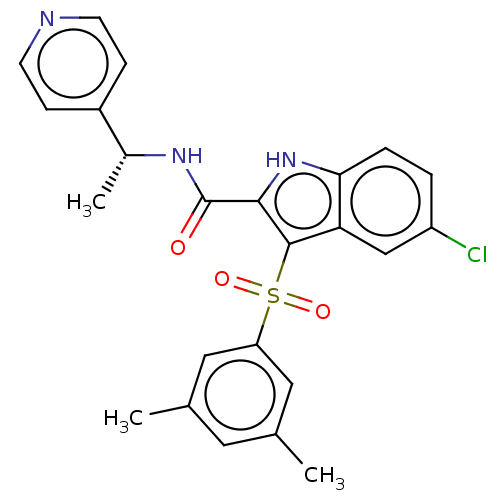 (CHEMBL3358176) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50497099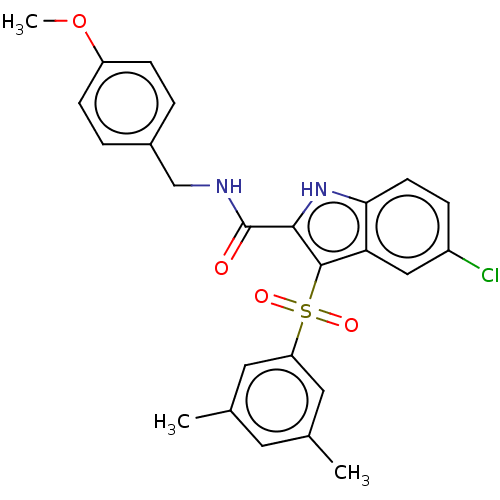 (CHEMBL3263459) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033865 (CHEMBL3358161) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase Y181I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556160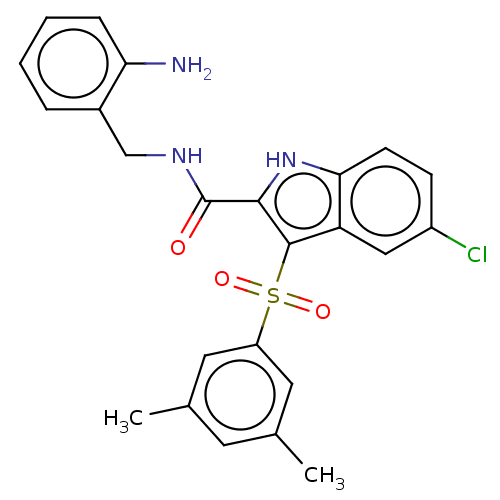 (CHEMBL4755777) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RNA-dependent DNA polymerase activity of recombinant HIV-1 p66/p51 reverse transcriptase K103N mutant assessed as inhibition of [3H]dTT... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50497108 (CHEMBL3263466) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033878 (CHEMBL3358170) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase K103N mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase V106A mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase V106A mutant using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RNA-dependent DNA polymerase activity of wild type recombinant HIV-1 His-tagged p66/p51 reverse transcriptase assessed as inhibition of... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 reverse transcriptase His-tagged p66/p51 associated RNA dependent DNA polymerase activity expressed in Escherichia coli asse... | J Med Chem 60: 6528-6547 (2017) Article DOI: 10.1021/acs.jmedchem.6b01906 BindingDB Entry DOI: 10.7270/Q28K7CTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50497092 (CHEMBL3263471) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase L1001 mutant using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556164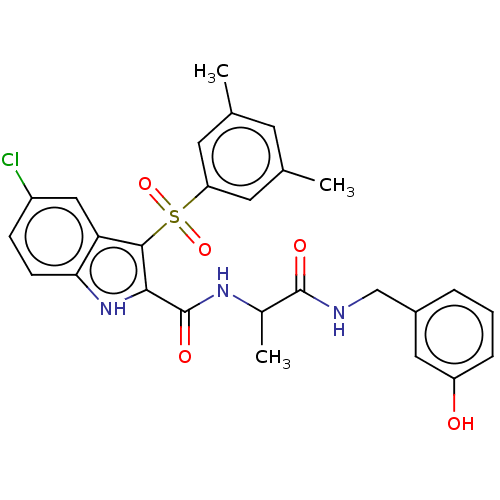 (CHEMBL4788245) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RNA-dependent DNA polymerase activity of recombinant HIV-1 p66/p51 reverse transcriptase K103N mutant assessed as inhibition of [3H]dTT... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase L1001 mutant using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033883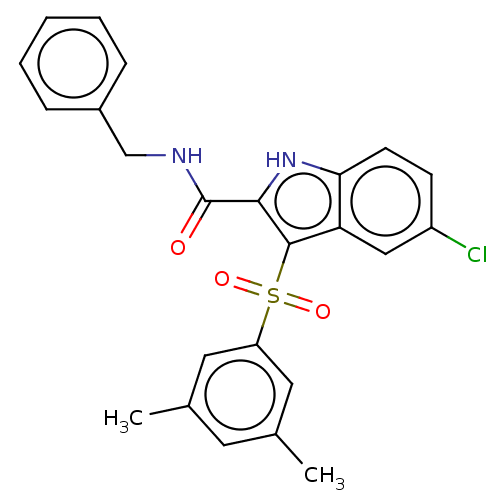 (CHEMBL1766221) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033877 (CHEMBL3358169) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033863 (CHEMBL3358176) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033864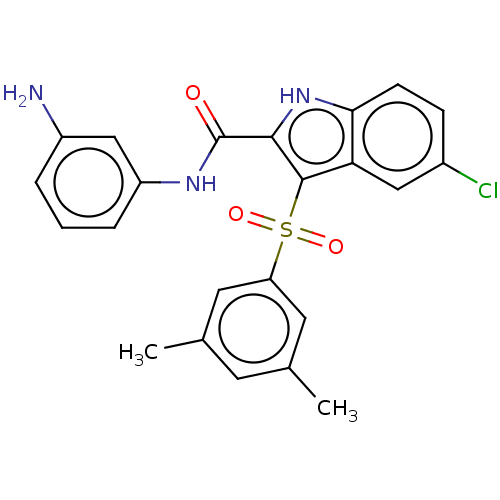 (CHEMBL3358160) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556160 (CHEMBL4755777) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RNA-dependent DNA polymerase activity of wild type recombinant HIV-1 His-tagged p66/p51 reverse transcriptase assessed as inhibition of... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033862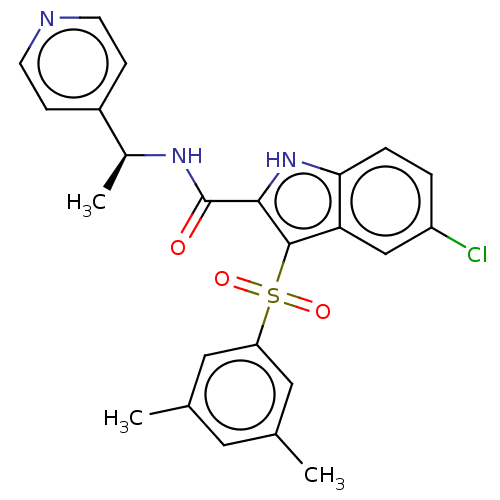 (CHEMBL3358175) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RNA-dependent DNA polymerase activity of recombinant HIV-1 p66/p51 reverse transcriptase K103N mutant assessed as inhibition of [3H]dTT... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase K103N mutant using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase K103N mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033875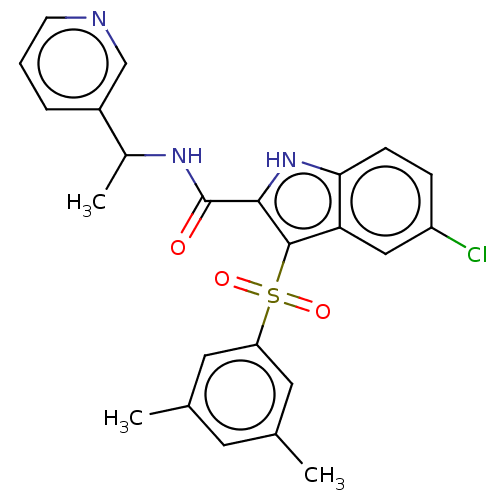 (CHEMBL3358167) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033883 (CHEMBL1766221) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 mi... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50497091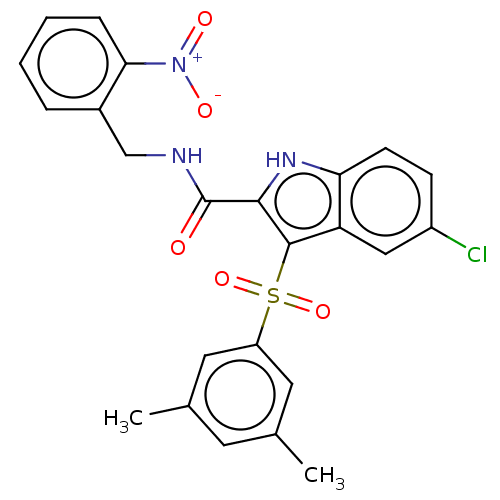 (CHEMBL3263464) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase K103N mutant using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50469959 (CHEMBL4068998) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 reverse transcriptase His-tagged p66/p51 associated RNA dependent DNA polymerase activity expressed in Escherichia coli asse... | J Med Chem 60: 6528-6547 (2017) Article DOI: 10.1021/acs.jmedchem.6b01906 BindingDB Entry DOI: 10.7270/Q28K7CTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured f... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50497091 (CHEMBL3263464) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50497101 (CHEMBL3263463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase K103N mutant using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033863 (CHEMBL3358176) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase V106A mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50497101 (CHEMBL3263463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033884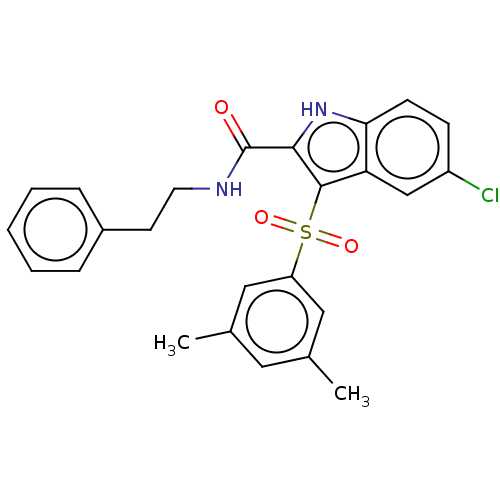 (CHEMBL1766233) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033880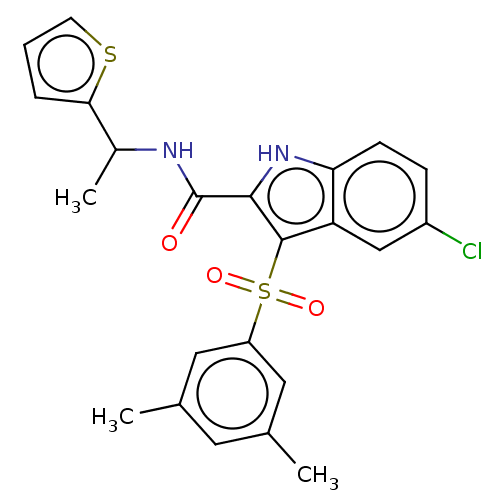 (CHEMBL3358172) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50497096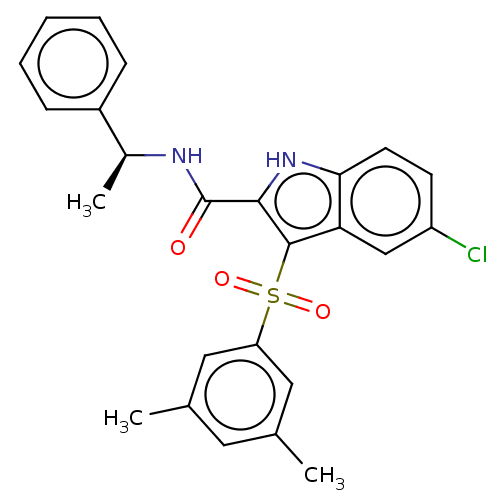 (CHEMBL3263479) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase V106A mutant using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033866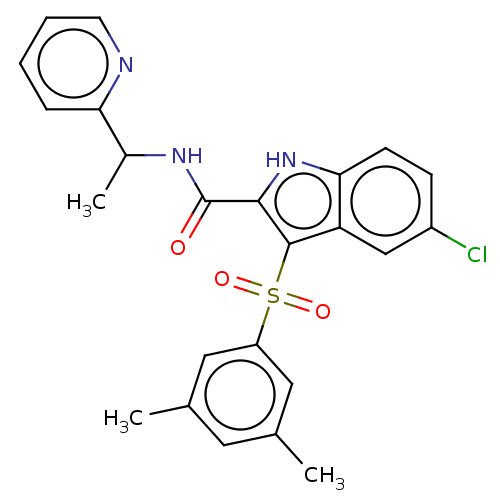 (CHEMBL3358166) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50497101 (CHEMBL3263463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of HIV1 wild-type reverse transcriptase L1001 mutant using [3H]dTTP by scintillation counting | Eur J Med Chem 80: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.04.027 BindingDB Entry DOI: 10.7270/Q2VM4G8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 286 total ) | Next | Last >> |