

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50075926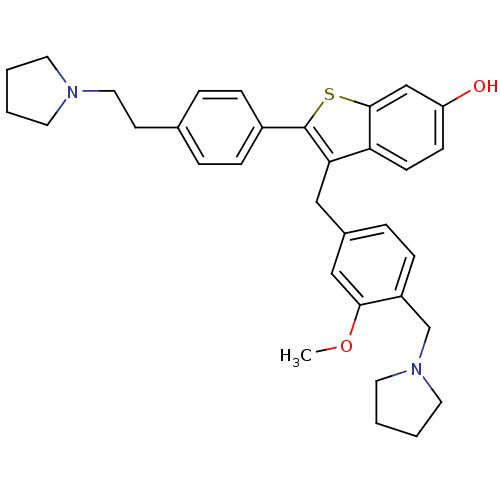 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075934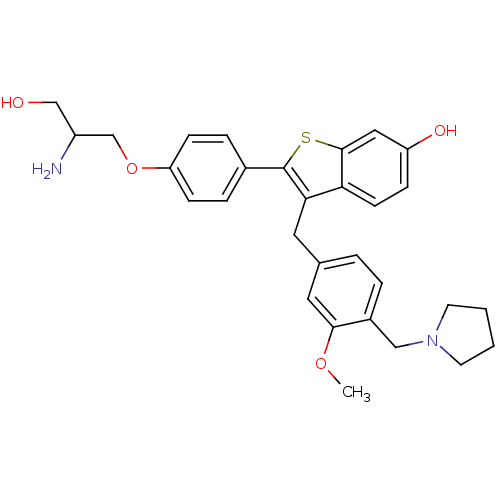 (2-[4-(2-Amino-3-hydroxy-propoxy)-phenyl]-3-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075937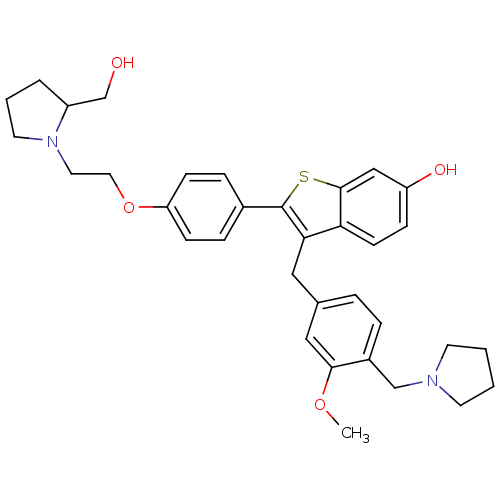 (2-{4-[2-(2-Hydroxymethyl-pyrrolidin-1-yl)-ethoxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075928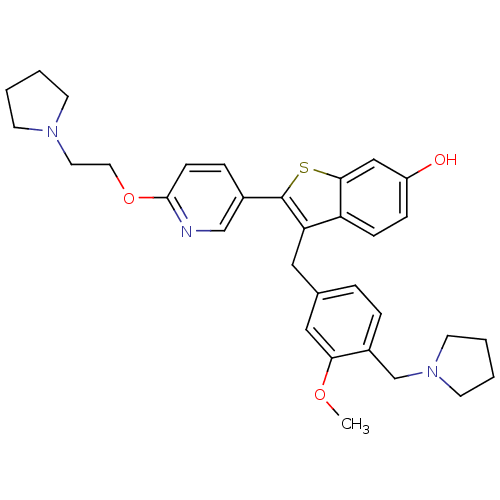 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50427452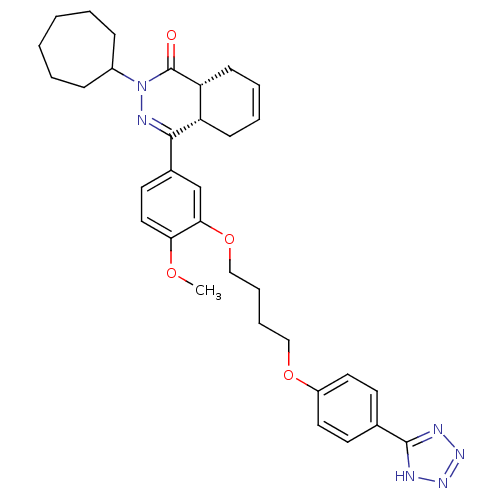 (CHEMBL2326941) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075938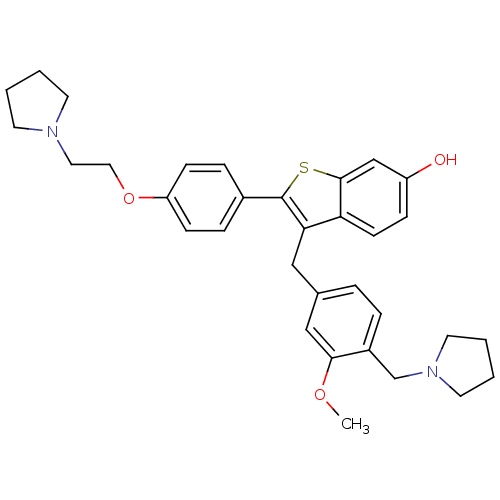 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075932 (2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-N-{4-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity towards alpha4-beta2 measured by using the inhibition of [3H]NIC binding to rat striatal membrane | Bioorg Med Chem Lett 13: 97-100 (2002) BindingDB Entry DOI: 10.7270/Q2736RF1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM315477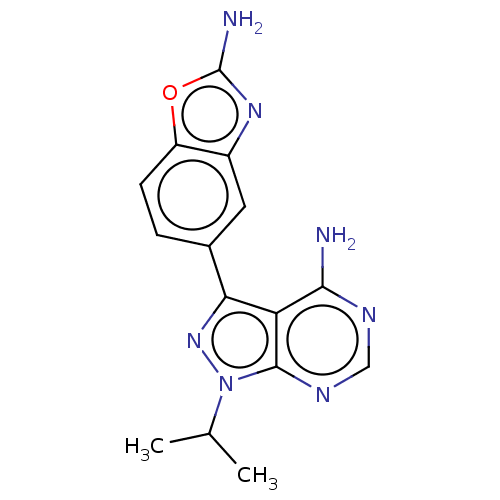 (US10172858, Table 1.1 | US10172858, Table 1.22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mTOR (unknown origin) by LanthaScreen assay | Citation and Details Article DOI: 10.1039/d0md00227e BindingDB Entry DOI: 10.7270/Q2G164F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075935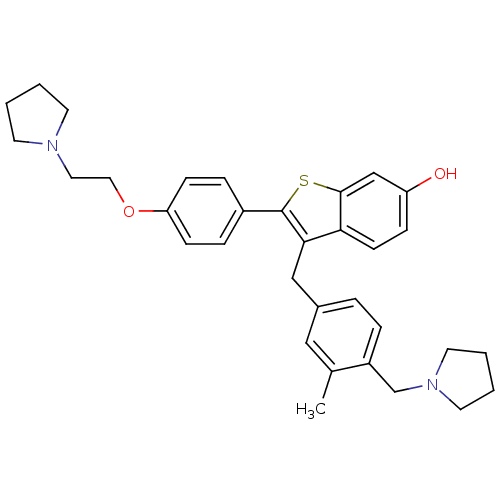 (3-(3-Methyl-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075931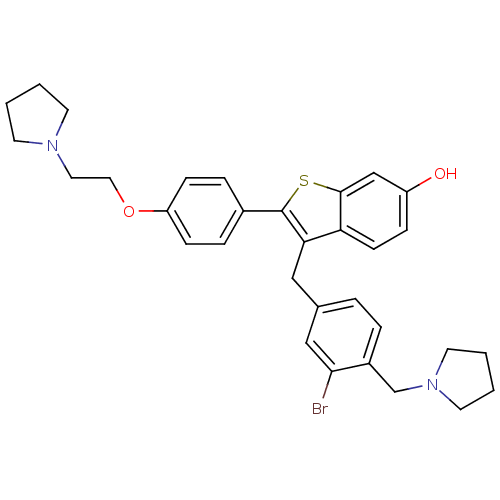 (3-(3-BROMO-4-PYRROLIDIN-1-YLMETHYL-BENZYL)-2-[4-PY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075939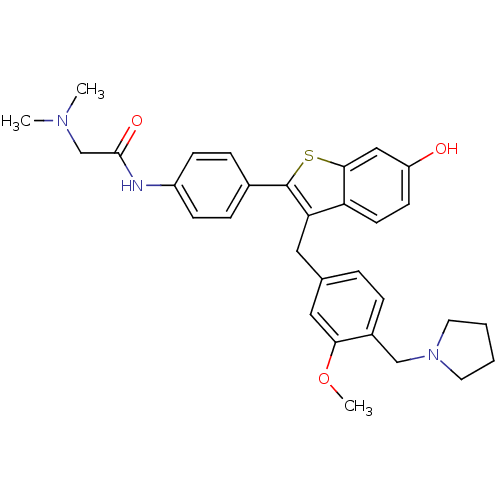 (2-Dimethylamino-N-{4-[6-hydroxy-3-(3-methoxy-4-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075927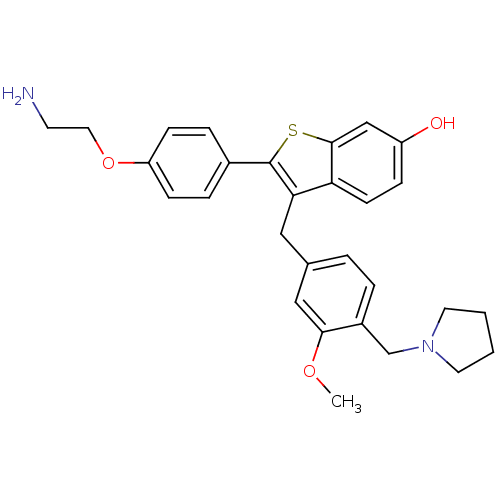 (2-[4-(2-Amino-ethoxy)-phenyl]-3-(3-methoxy-4-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50427452 (CHEMBL2326941) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length Trypanosoma brucei PDEB1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075930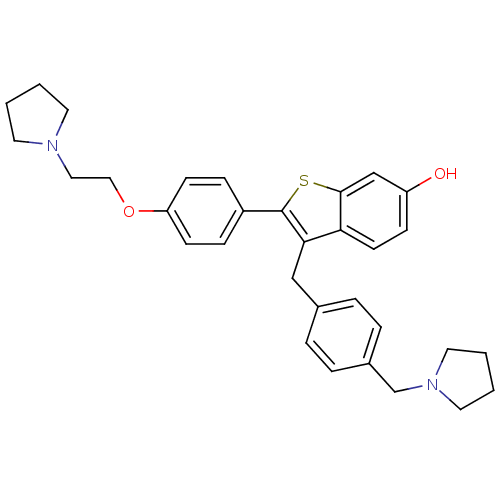 (2-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenyl]-3-(4-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50366779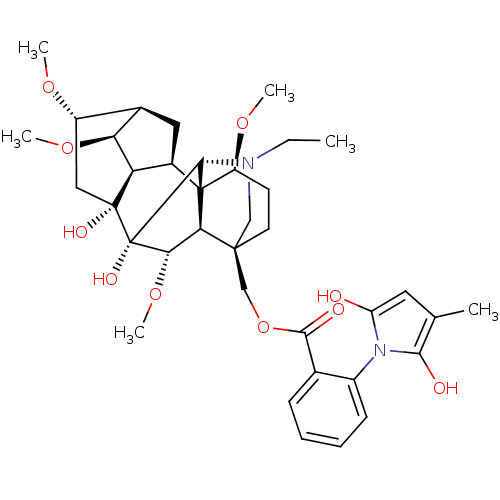 (METHYLLYCACONITINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity towards alpha-7 neuronal nicotonic acetylcholine receptor (nAChRs) measured by using the inhibition of [3H]-MLA binding to whole bra... | Bioorg Med Chem Lett 13: 97-100 (2002) BindingDB Entry DOI: 10.7270/Q2736RF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075933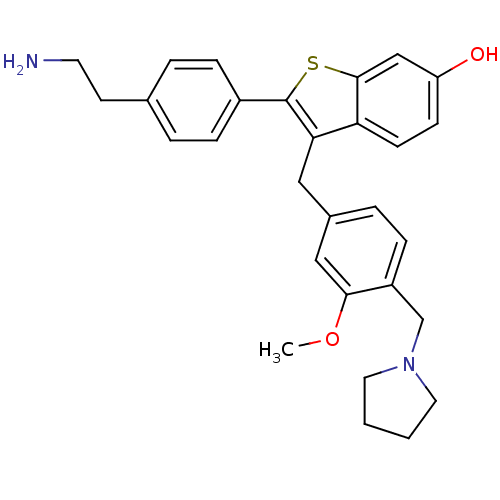 (2-[4-(2-Amino-ethyl)-phenyl]-3-(3-methoxy-4-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075941 (3-(3-Hydroxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50121739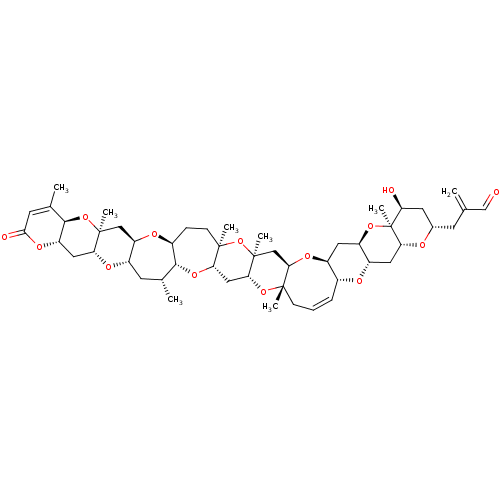 (Bungarotoxin Neuronal | Bungarotoxin,Alpha | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity towards alpha-7 neuronal nicotonic acetylcholine receptor (nAChRs) measured by using the inhibition of [3H]-MLA binding to whole bra... | Bioorg Med Chem Lett 13: 97-100 (2002) BindingDB Entry DOI: 10.7270/Q2736RF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226932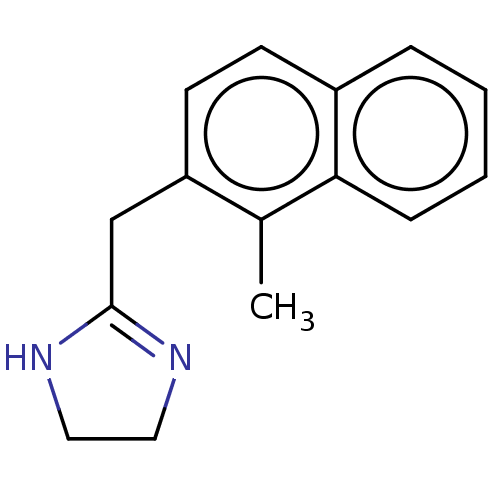 (CHEMBL559829) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226924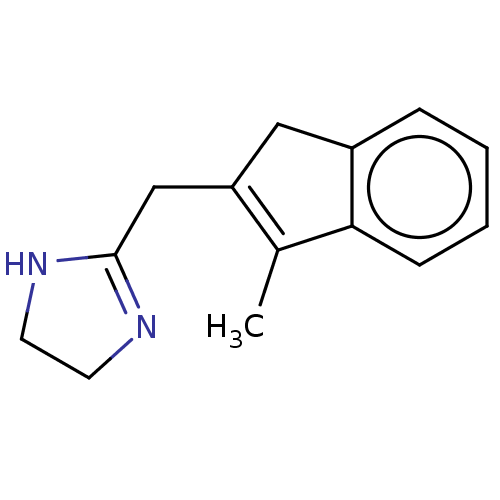 (CHEMBL545647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075929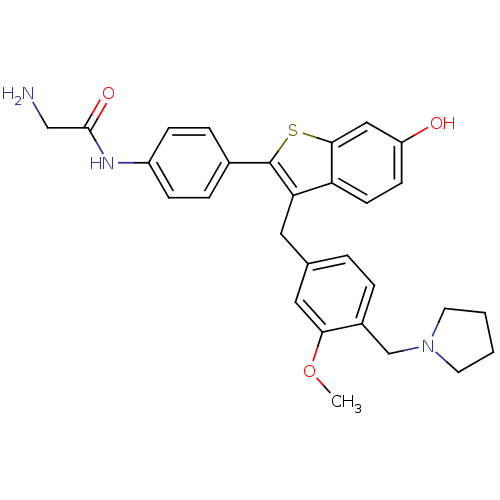 (2-Amino-N-{4-[6-hydroxy-3-(3-methoxy-4-pyrrolidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075940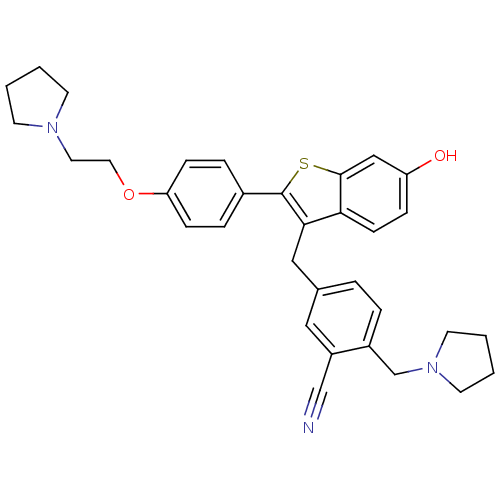 (5-{6-Hydroxy-2-[4-(2-pyrrolidin-1-yl-ethoxy)-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50019848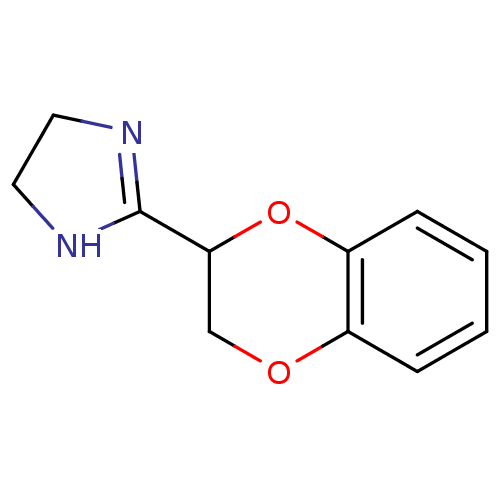 (2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]-clonidine from alpha-1 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50019848 (2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075936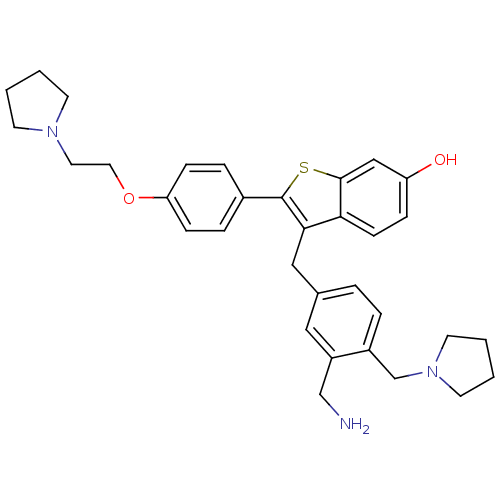 (3-(3-Aminomethyl-4-pyrrolidin-1-ylmethyl-benzyl)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226929 (CHEMBL545646) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226929 (CHEMBL545646) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50454831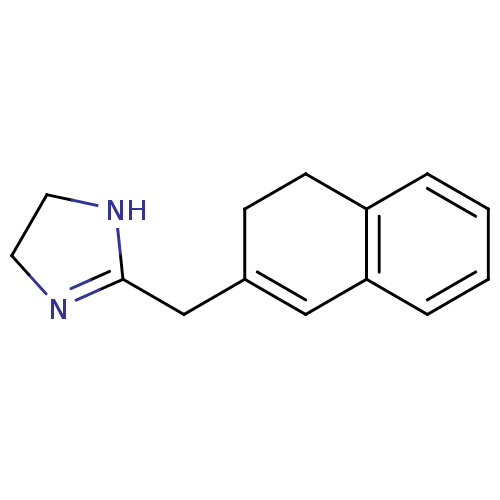 (Napamezole) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50454831 (Napamezole) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50453147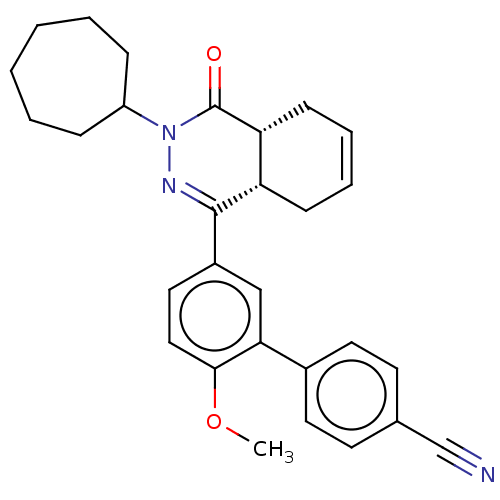 (CHEMBL4202704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226928 (CHEMBL553439) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50035415 ((S)-1'-demethylnicotine | 1'-demethyl nicotine | 3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity towards alpha4-beta2 measured by using the inhibition of [3H]NIC binding to rat striatal membrane | Bioorg Med Chem Lett 13: 97-100 (2002) BindingDB Entry DOI: 10.7270/Q2736RF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50453158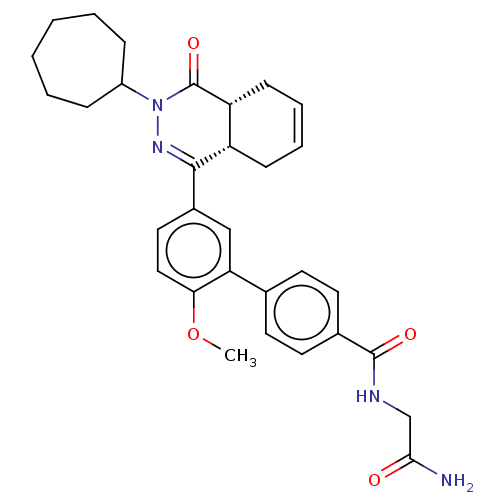 (CHEMBL4210475) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length Trypanosoma brucei PDEB1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50453158 (CHEMBL4210475) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length Trypanosoma brucei PDEB1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50057120 (2-Naphthalen-2-ylmethyl-4,5-dihydro-1H-imidazole |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50222218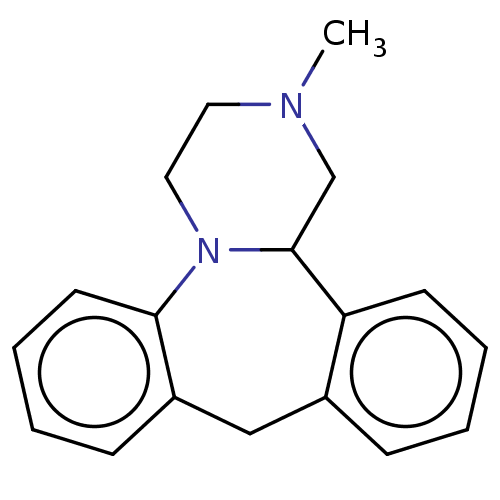 (CHEBI:51137 | Mianserin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226927 (CHEMBL544701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50454831 (Napamezole) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from alpha-1 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226930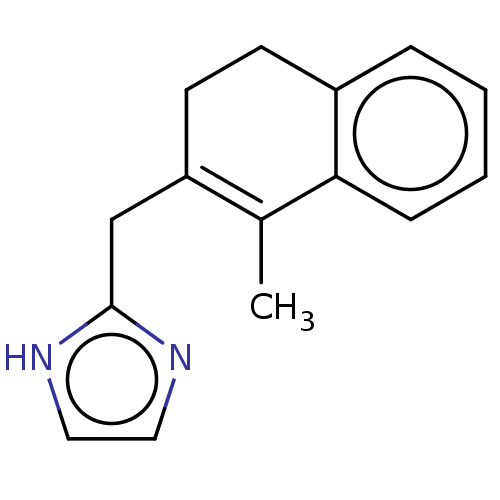 (CHEMBL35345) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50453161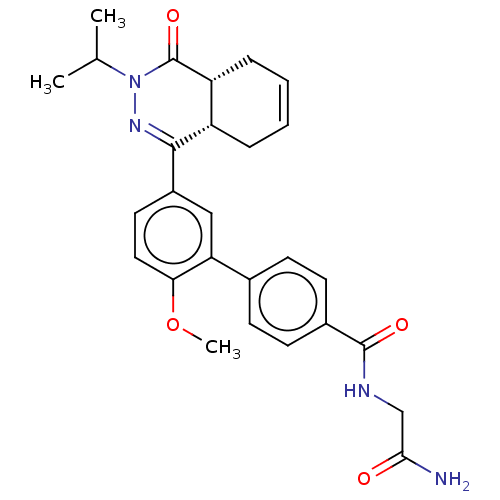 (CHEMBL4204999) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length Trypanosoma brucei PDEB1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226926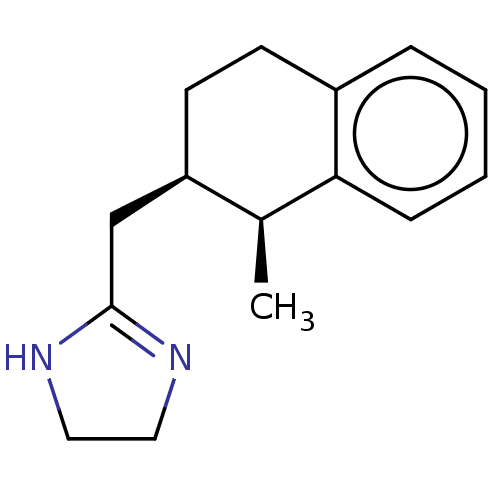 (CHEMBL544706) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50226929 (CHEMBL545646) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from alpha-1 adrenergic receptor in rat brain homogenates in vitro | J Med Chem 30: 1482-9 (1987) BindingDB Entry DOI: 10.7270/Q2WS8WGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50453171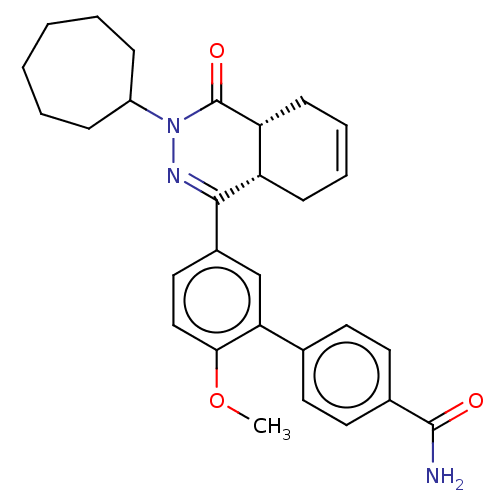 (CHEMBL4202975) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length Trypanosoma brucei PDEB1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50453171 (CHEMBL4202975) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 175 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | Biochemistry 41: 10810-8 (2002) Article DOI: 10.1021/bi020151+ BindingDB Entry DOI: 10.7270/Q2HQ3X52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50453180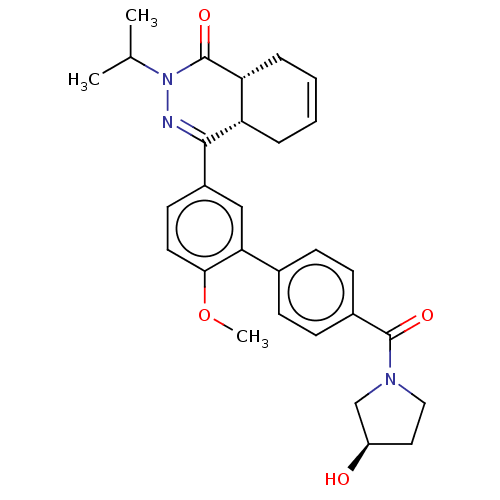 (CHEMBL4208363) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length Trypanosoma brucei PDEB1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 843 total ) | Next | Last >> |