

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070501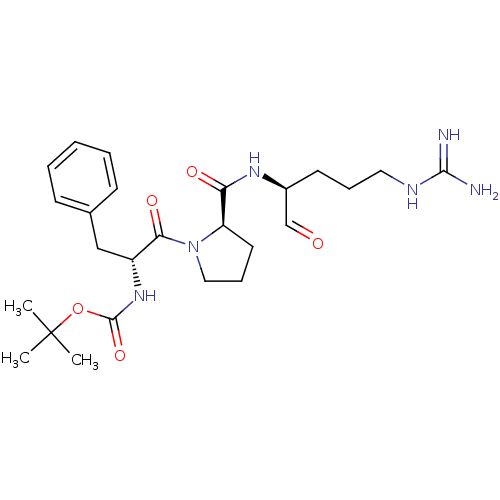 (CHEMBL417682 | {(R)-1-Benzyl-2-[(R)-2-((S)-1-formy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit Trypsin was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070501 (CHEMBL417682 | {(R)-1-Benzyl-2-[(R)-2-((S)-1-formy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit Thrombin was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50070499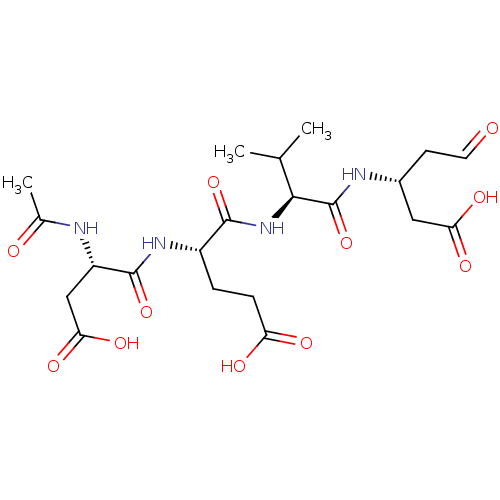 ((R)-3-{(S)-2-[(S)-2-((S)-2-Acetylamino-3-carboxy-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Amidolytic activity of THP.1 cell lysate against IL-1 beta converting enzyme using C1-Asp-AMC as a substrate | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50366497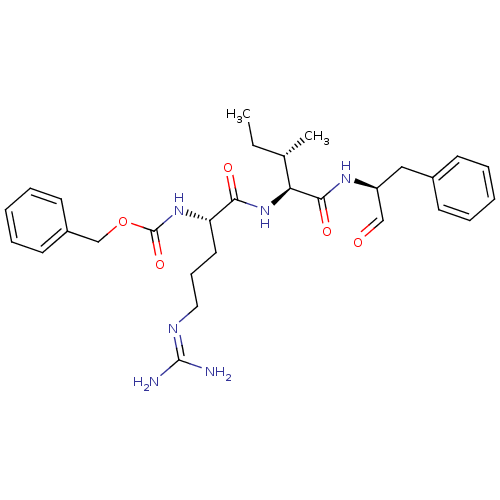 (CHEMBL1790164) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit papain was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50070495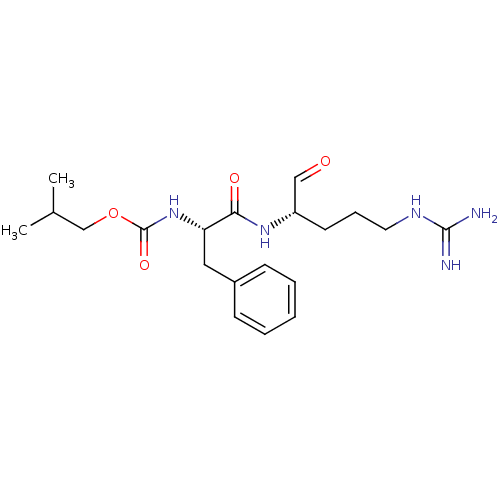 (CHEMBL290218 | [(S)-1-((S)-1-Formyl-4-guanidino-bu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit papain was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070502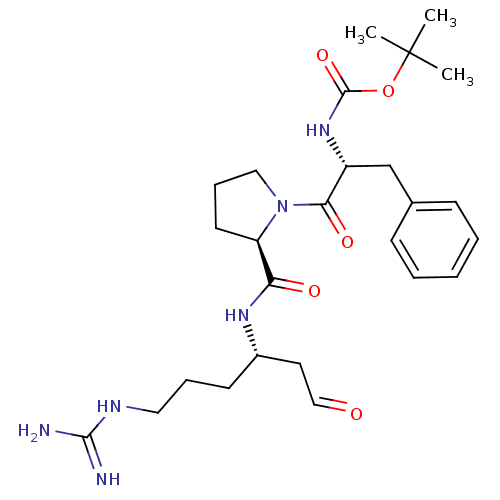 (((R)-1-Benzyl-2-{(R)-2-[(S)-4-guanidino-1-(2-oxo-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit Thrombin was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50070499 ((R)-3-{(S)-2-[(S)-2-((S)-2-Acetylamino-3-carboxy-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 438 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Amidolytic activity of THP.1 cell lysate against Caspase-3 using C3-Asp-AMC as a substrate | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070502 (((R)-1-Benzyl-2-{(R)-2-[(S)-4-guanidino-1-(2-oxo-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit Trypsin was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070494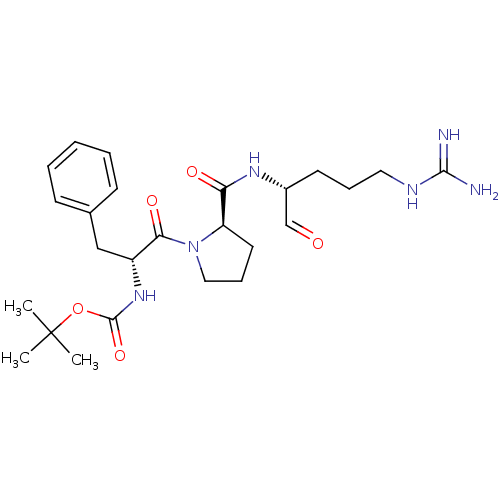 (CHEMBL36336 | {(R)-1-Benzyl-2-[(R)-2-((R)-1-formyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit Trypsin was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM10355 ((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit IL-1 beta converting enzyme was evaluated in LPS-stimulated human whole blood | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50070496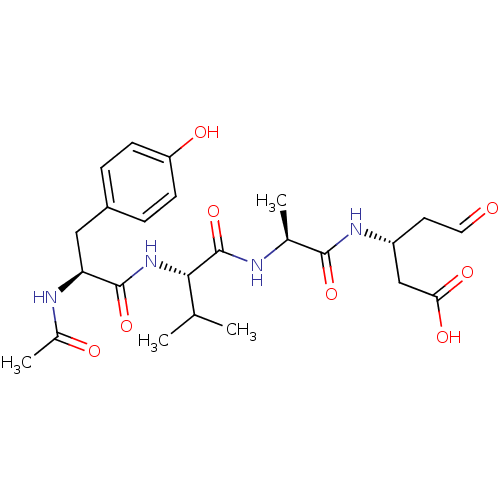 ((R)-3-((S)-2-{(S)-2-[(S)-2-Acetylamino-3-(4-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit IL-1 beta converting enzyme was evaluated in LPS-stimulated human whole blood | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070494 (CHEMBL36336 | {(R)-1-Benzyl-2-[(R)-2-((R)-1-formyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit Thrombin was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50366496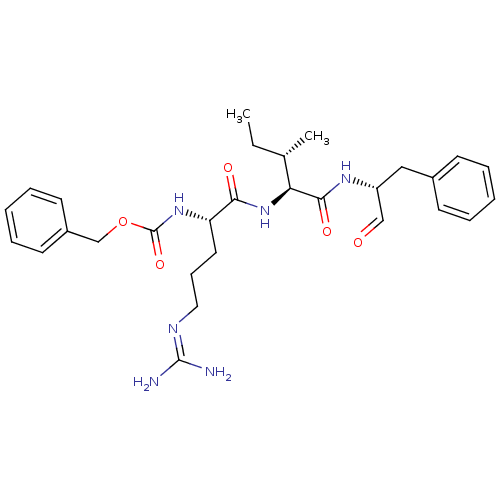 (CHEMBL1790166) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit papain was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50070503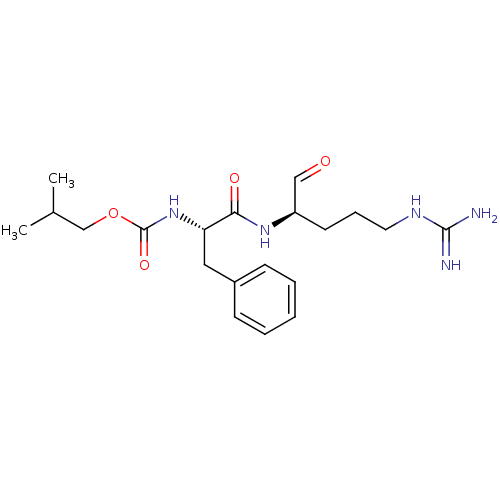 (CHEMBL286910 | [(S)-1-((R)-1-Formyl-4-guanidino-bu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit papain was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50366498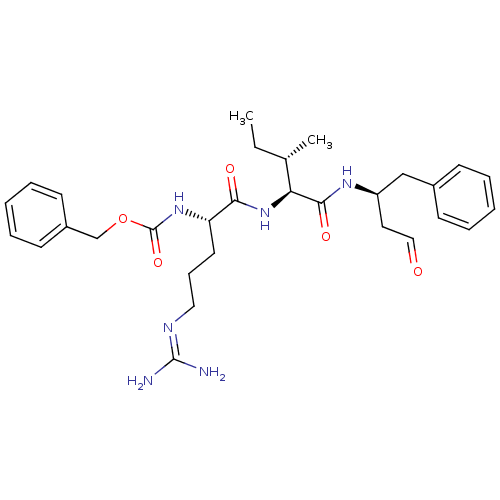 (CHEMBL1790165) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit papain was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM10355 ((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Amidolytic activity of THP.1 cell lysate against IL-1 beta converting enzyme using C1-Asp-AMC as a substrate | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM10246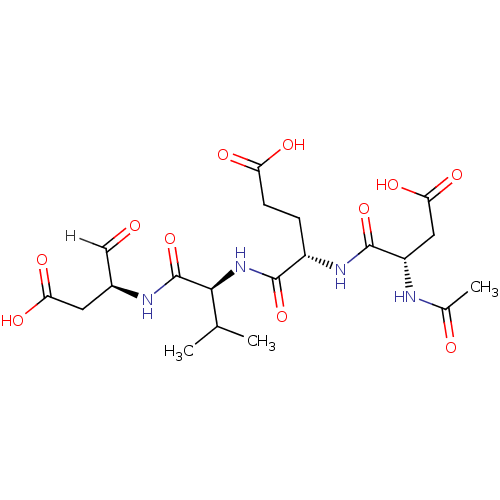 ((4S)-4-{[(1S)-1-{[(2S)-1-carboxy-3-oxopropan-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Amidolytic activity of THP.1 cell lysate against IL-1 beta converting enzyme using C1-Asp-AMC as a substrate | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50070497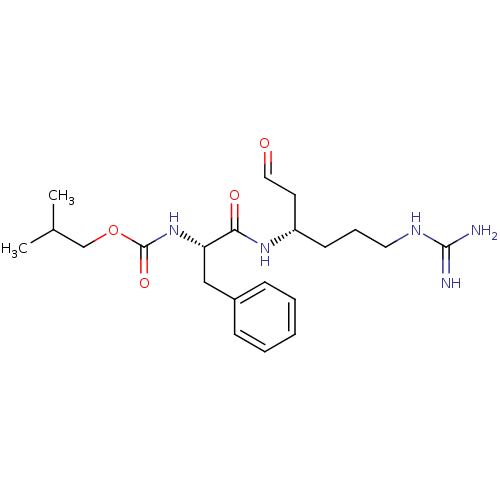 (CHEMBL37975 | {(S)-1-[(S)-4-Guanidino-1-(2-oxo-eth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Ability to inhibit papain was evaluated by amidolytic method using fluorogenic or chromogenic substrates | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50070496 ((R)-3-((S)-2-{(S)-2-[(S)-2-Acetylamino-3-(4-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Amidolytic activity of THP.1 cell lysate against IL-1 beta converting enzyme using C1-Asp-AMC as a substrate | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10355 ((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Research Curated by ChEMBL | Assay Description Amidolytic activity of THP.1 cell lysate against Caspase-3 using C3-Asp-AMC as a substrate | Bioorg Med Chem Lett 8: 1477-82 (1999) Checked by Author BindingDB Entry DOI: 10.7270/Q2GB24KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||