 Found 1030 hits with Last Name = 'barnes' and Initial = 'd'
Found 1030 hits with Last Name = 'barnes' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451155
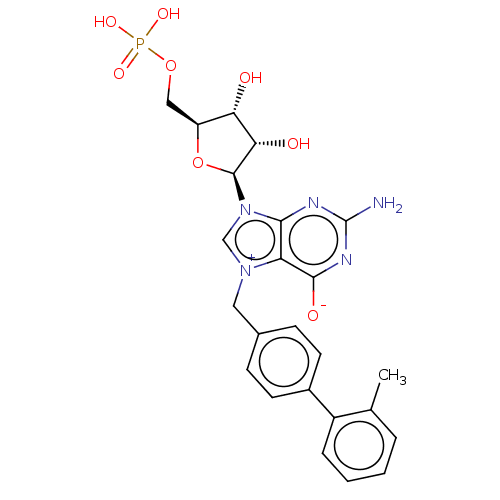
(US10676499, Example 49)Show SMILES Cc1ccccc1-c1ccc(C[n+]2cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c3nc(N)nc([O-])c23)cc1 |r| Show InChI InChI=1S/C24H26N5O8P/c1-13-4-2-3-5-16(13)15-8-6-14(7-9-15)10-28-12-29(21-18(28)22(32)27-24(25)26-21)23-20(31)19(30)17(37-23)11-36-38(33,34)35/h2-9,12,17,19-20,23,30-31H,10-11H2,1H3,(H4-,25,26,27,32,33,34,35)/t17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451159
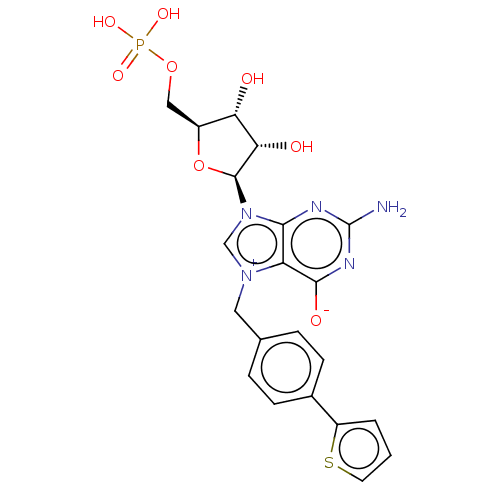
(US10676499, Example 57)Show SMILES Nc1nc([O-])c2[n+](Cc3ccc(cc3)-c3cccs3)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C21H22N5O8PS/c22-21-23-18-15(19(29)24-21)25(8-11-3-5-12(6-4-11)14-2-1-7-36-14)10-26(18)20-17(28)16(27)13(34-20)9-33-35(30,31)32/h1-7,10,13,16-17,20,27-28H,8-9H2,(H4-,22,23,24,29,30,31,32)/t13-,16-,17-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451145
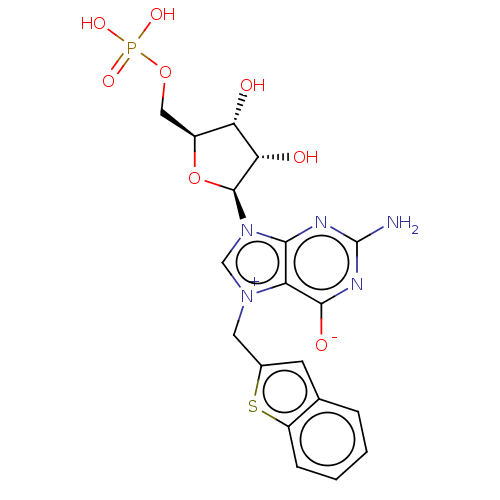
(US10676499, Example 17)Show SMILES Nc1nc([O-])c2[n+](Cc3cc4ccccc4s3)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C19H20N5O8PS/c20-19-21-16-13(17(27)22-19)23(6-10-5-9-3-1-2-4-12(9)34-10)8-24(16)18-15(26)14(25)11(32-18)7-31-33(28,29)30/h1-5,8,11,14-15,18,25-26H,6-7H2,(H4-,20,21,22,27,28,29,30)/t11-,14-,15-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451148
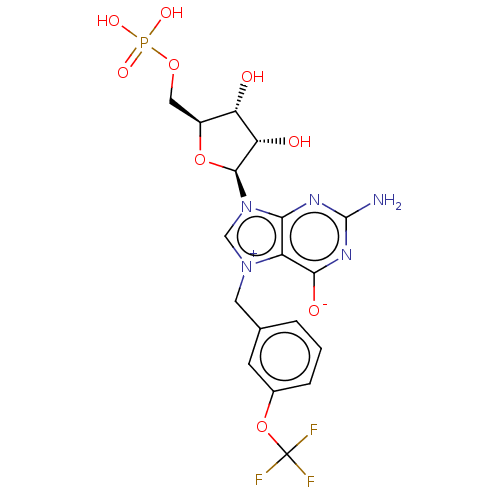
(US10676499, Example 29)Show SMILES Nc1nc([O-])c2[n+](Cc3cccc(OC(F)(F)F)c3)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C18H19F3N5O9P/c19-18(20,21)35-9-3-1-2-8(4-9)5-25-7-26(14-11(25)15(29)24-17(22)23-14)16-13(28)12(27)10(34-16)6-33-36(30,31)32/h1-4,7,10,12-13,16,27-28H,5-6H2,(H4-,22,23,24,29,30,31,32)/t10-,12-,13-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451158

(US10676499, Example 55)Show SMILES Nc1nc([O-])c2[n+](Cc3ccc(c(Cl)c3)-c3ccccc3)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C23H23ClN5O8P/c24-15-8-12(6-7-14(15)13-4-2-1-3-5-13)9-28-11-29(20-17(28)21(32)27-23(25)26-20)22-19(31)18(30)16(37-22)10-36-38(33,34)35/h1-8,11,16,18-19,22,30-31H,9-10H2,(H4-,25,26,27,32,33,34,35)/t16-,18-,19-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451147
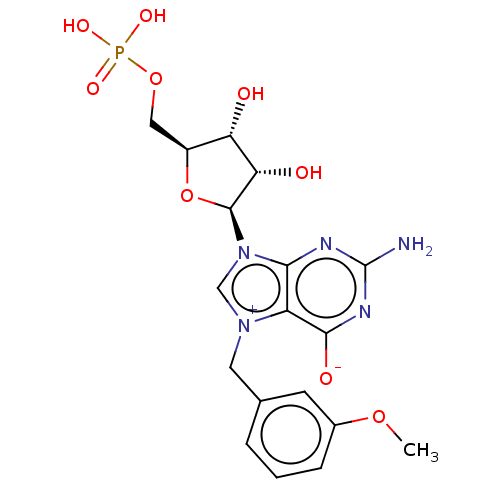
(US10676499, Example 26)Show SMILES COc1cccc(C[n+]2cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c3nc(N)nc([O-])c23)c1 |r| Show InChI InChI=1S/C18H22N5O9P/c1-30-10-4-2-3-9(5-10)6-22-8-23(15-12(22)16(26)21-18(19)20-15)17-14(25)13(24)11(32-17)7-31-33(27,28)29/h2-5,8,11,13-14,17,24-25H,6-7H2,1H3,(H4-,19,20,21,26,27,28,29)/t11-,13-,14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451144
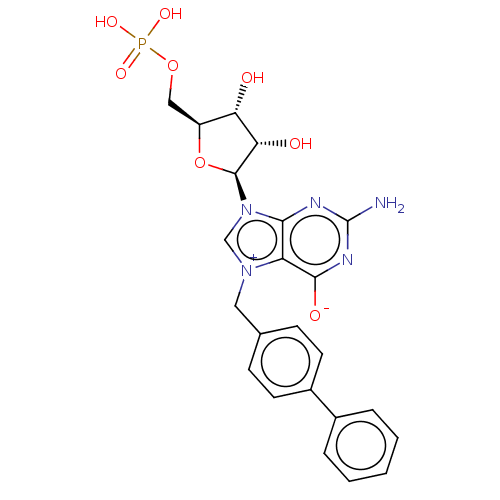
(US10676499, Example 1)Show SMILES Nc1nc([O-])c2[n+](Cc3ccc(cc3)-c3ccccc3)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C23H24N5O8P/c24-23-25-20-17(21(31)26-23)27(10-13-6-8-15(9-7-13)14-4-2-1-3-5-14)12-28(20)22-19(30)18(29)16(36-22)11-35-37(32,33)34/h1-9,12,16,18-19,22,29-30H,10-11H2,(H4-,24,25,26,31,32,33,34)/t16-,18-,19-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451150
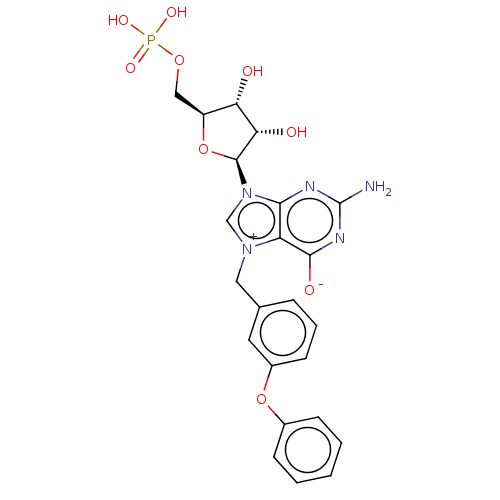
(US10676499, Example 33)Show SMILES Nc1nc([O-])c2[n+](Cc3cccc(Oc4ccccc4)c3)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C23H24N5O9P/c24-23-25-20-17(21(31)26-23)27(10-13-5-4-8-15(9-13)36-14-6-2-1-3-7-14)12-28(20)22-19(30)18(29)16(37-22)11-35-38(32,33)34/h1-9,12,16,18-19,22,29-30H,10-11H2,(H4-,24,25,26,31,32,33,34)/t16-,18-,19-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451146

(US10676499, Example 18)Show SMILES CO[C@H]1[C@@H](O)[C@H](COP(O)(O)=O)O[C@@H]1n1c[n+](CCOc2ccc(Cl)cc2)c2c([O-])nc(N)nc12 |r| Show InChI InChI=1S/C19H23ClN5O9P/c1-31-15-14(26)12(8-33-35(28,29)30)34-18(15)25-9-24(13-16(25)22-19(21)23-17(13)27)6-7-32-11-4-2-10(20)3-5-11/h2-5,9,12,14-15,18,26H,6-8H2,1H3,(H4-,21,22,23,27,28,29,30)/t12-,14-,15-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451156
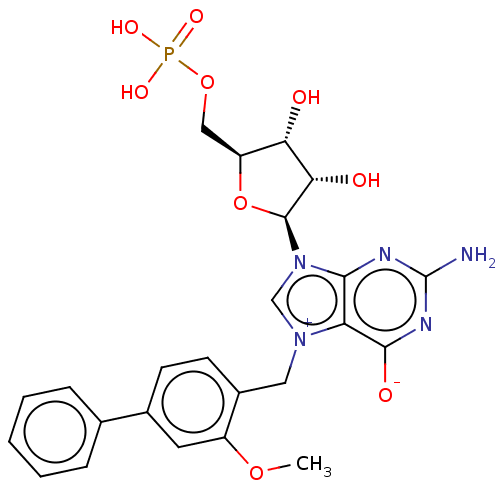
(US10676499, Example 53)Show SMILES COc1cc(ccc1C[n+]1cn([C@H]2O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]2O)c2nc(N)nc([O-])c12)-c1ccccc1 |r| Show InChI InChI=1S/C24H26N5O9P/c1-36-16-9-14(13-5-3-2-4-6-13)7-8-15(16)10-28-12-29(21-18(28)22(32)27-24(25)26-21)23-20(31)19(30)17(38-23)11-37-39(33,34)35/h2-9,12,17,19-20,23,30-31H,10-11H2,1H3,(H4-,25,26,27,32,33,34,35)/t17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50113399
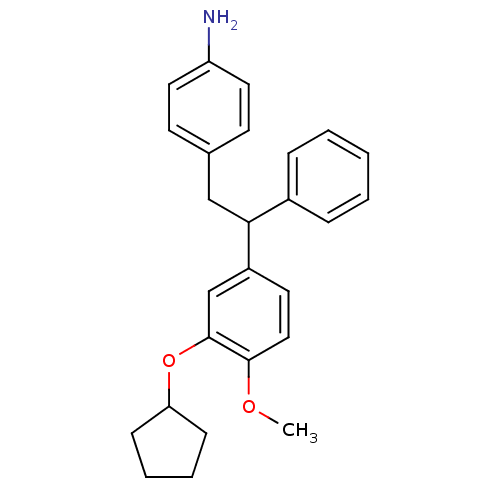
(4-[2-(3-Cyclopentyloxy-4-methoxy-phenyl)-2-phenyl-...)Show SMILES COc1ccc(cc1OC1CCCC1)C(Cc1ccc(N)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-28-25-16-13-21(18-26(25)29-23-9-5-6-10-23)24(20-7-3-2-4-8-20)17-19-11-14-22(27)15-12-19/h2-4,7-8,11-16,18,23-24H,5-6,9-10,17,27H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 423 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant phosphodiesterase 4A |
Bioorg Med Chem Lett 12: 1451-6 (2002)
BindingDB Entry DOI: 10.7270/Q22J6B5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM249466
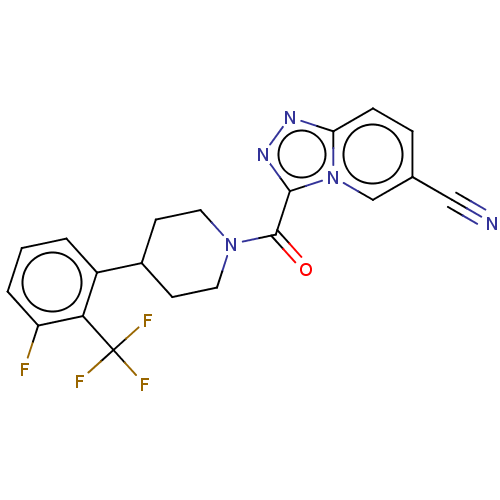
(US10072016, Compound 65 | US10407433, Compound 65 ...)Show SMILES Fc1cccc(C2CCN(CC2)C(=O)c2nnc3ccc(cn23)C#N)c1C(F)(F)F Show InChI InChI=1S/C20H15F4N5O/c21-15-3-1-2-14(17(15)20(22,23)24)13-6-8-28(9-7-13)19(30)18-27-26-16-5-4-12(10-25)11-29(16)18/h1-5,11,13H,6-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP2D6 in human liver microsomes assessed as equilibrium inhibition binding constant in presence of NADPH by LC-MS/MS an... |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451160
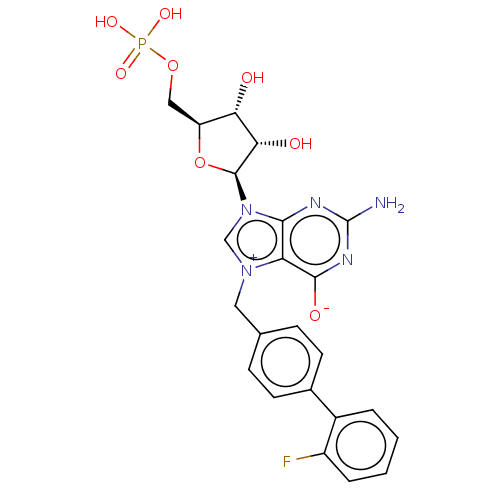
(US10676499, Example 58)Show SMILES Nc1nc([O-])c2[n+](Cc3ccc(cc3)-c3ccccc3F)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C23H23FN5O8P/c24-15-4-2-1-3-14(15)13-7-5-12(6-8-13)9-28-11-29(20-17(28)21(32)27-23(25)26-20)22-19(31)18(30)16(37-22)10-36-38(33,34)35/h1-8,11,16,18-19,22,30-31H,9-10H2,(H4-,25,26,27,32,33,34,35)/t16-,18-,19-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50113373
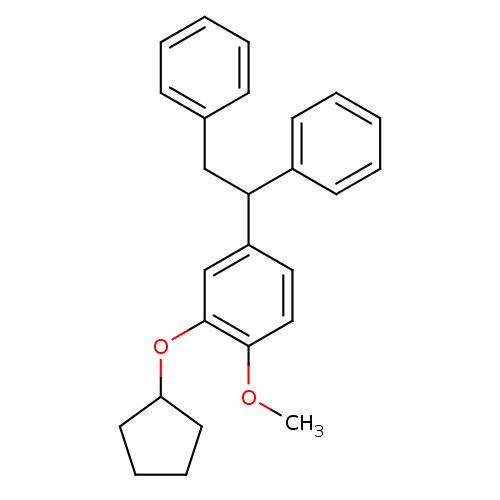
(2-Cyclopentyloxy-4-(1,2-diphenyl-ethyl)-1-methoxy-...)Show InChI InChI=1S/C26H28O2/c1-27-25-17-16-22(19-26(25)28-23-14-8-9-15-23)24(21-12-6-3-7-13-21)18-20-10-4-2-5-11-20/h2-7,10-13,16-17,19,23-24H,8-9,14-15,18H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant phosphodiesterase 4A |
Bioorg Med Chem Lett 12: 1451-6 (2002)
BindingDB Entry DOI: 10.7270/Q22J6B5D |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451151

(US10676499, Example 40)Show SMILES COC(=O)c1sccc1NC(=O)c1cccc(C[n+]2cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c3nc(N)nc([O-])c23)c1 |r| Show InChI InChI=1S/C24H25N6O11PS/c1-39-23(35)18-13(5-6-43-18)26-20(33)12-4-2-3-11(7-12)8-29-10-30(19-15(29)21(34)28-24(25)27-19)22-17(32)16(31)14(41-22)9-40-42(36,37)38/h2-7,10,14,16-17,22,31-32H,8-9H2,1H3,(H5-,25,26,27,28,33,34,35,36,37,38)/t14-,16-,17-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451157
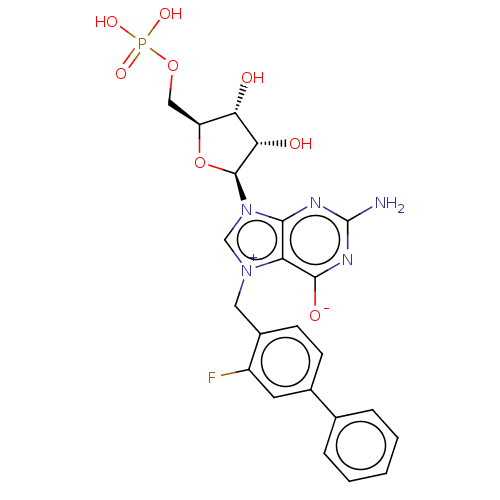
(US10676499, Example 54)Show SMILES Nc1nc([O-])c2[n+](Cc3ccc(cc3F)-c3ccccc3)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C23H23FN5O8P/c24-15-8-13(12-4-2-1-3-5-12)6-7-14(15)9-28-11-29(20-17(28)21(32)27-23(25)26-20)22-19(31)18(30)16(37-22)10-36-38(33,34)35/h1-8,11,16,18-19,22,30-31H,9-10H2,(H4-,25,26,27,32,33,34,35)/t16-,18-,19-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50501900

(CHEMBL4463039)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)c1nnc2ccc(cn12)C#N Show InChI InChI=1S/C20H16F3N5O/c21-20(22,23)16-4-2-1-3-15(16)14-7-9-27(10-8-14)19(29)18-26-25-17-6-5-13(11-24)12-28(17)18/h1-6,12,14H,7-10H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP2D6 in human liver microsomes assessed as equilibrium inhibition binding constant in presence of NADPH by LC-MS/MS an... |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451154

(US10676499, Example 43)Show SMILES Nc1nc([O-])c2[n+](Cc3cccc(OCc4ccccc4)c3)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C24H26N5O9P/c25-24-26-21-18(22(32)27-24)28(10-15-7-4-8-16(9-15)36-11-14-5-2-1-3-6-14)13-29(21)23-20(31)19(30)17(38-23)12-37-39(33,34)35/h1-9,13,17,19-20,23,30-31H,10-12H2,(H4-,25,26,27,32,33,34,35)/t17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451153
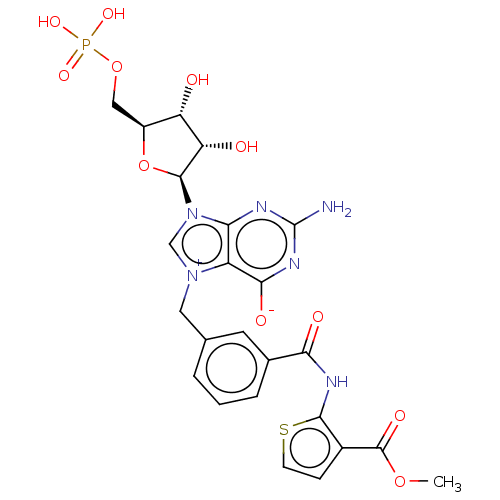
(US10676499, Example 42)Show SMILES COC(=O)c1ccsc1NC(=O)c1cccc(C[n+]2cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c3nc(N)nc([O-])c23)c1 |r| Show InChI InChI=1S/C24H25N6O11PS/c1-39-23(35)13-5-6-43-21(13)27-19(33)12-4-2-3-11(7-12)8-29-10-30(18-15(29)20(34)28-24(25)26-18)22-17(32)16(31)14(41-22)9-40-42(36,37)38/h2-7,10,14,16-17,22,31-32H,8-9H2,1H3,(H5-,25,26,27,28,33,34,35,36,37,38)/t14-,16-,17-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50113366
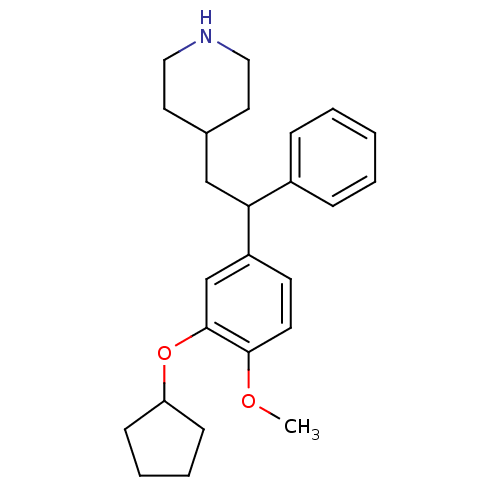
(4-[2-(3-Cyclopentyloxy-4-methoxy-phenyl)-2-phenyl-...)Show InChI InChI=1S/C25H33NO2/c1-27-24-12-11-21(18-25(24)28-22-9-5-6-10-22)23(20-7-3-2-4-8-20)17-19-13-15-26-16-14-19/h2-4,7-8,11-12,18-19,22-23,26H,5-6,9-10,13-17H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant phosphodiesterase 4A |
Bioorg Med Chem Lett 12: 1451-6 (2002)
BindingDB Entry DOI: 10.7270/Q22J6B5D |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451152
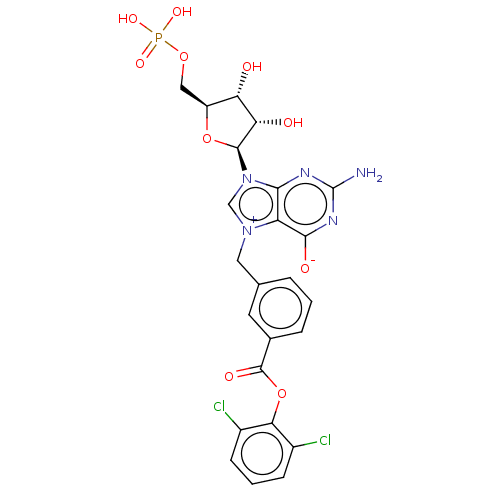
(US10676499, Example 41)Show SMILES Nc1nc([O-])c2[n+](Cc3cccc(c3)C(=O)Oc3c(Cl)cccc3Cl)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C24H22Cl2N5O10P/c25-13-5-2-6-14(26)19(13)41-23(35)12-4-1-3-11(7-12)8-30-10-31(20-16(30)21(34)29-24(27)28-20)22-18(33)17(32)15(40-22)9-39-42(36,37)38/h1-7,10,15,17-18,22,32-33H,8-9H2,(H4-,27,28,29,34,36,37,38)/t15-,17-,18-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 8.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM451149

(US10676499, Example 32)Show SMILES Nc1nc([O-])c2[n+](Cc3ccccc3CS(=O)(=O)c3ccccc3)cn([C@H]3O[C@@H](COP(O)(O)=O)[C@H](O)[C@@H]3O)c2n1 |r| Show InChI InChI=1S/C24H26N5O10PS/c25-24-26-21-18(22(32)27-24)28(13-29(21)23-20(31)19(30)17(39-23)11-38-40(33,34)35)10-14-6-4-5-7-15(14)12-41(36,37)16-8-2-1-3-9-16/h1-9,13,17,19-20,23,30-31H,10-12H2,(H4-,25,26,27,32,33,34,35)/t17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 9.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... |
US Patent US10676499 (2020)
BindingDB Entry DOI: 10.7270/Q2K64N44 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378304
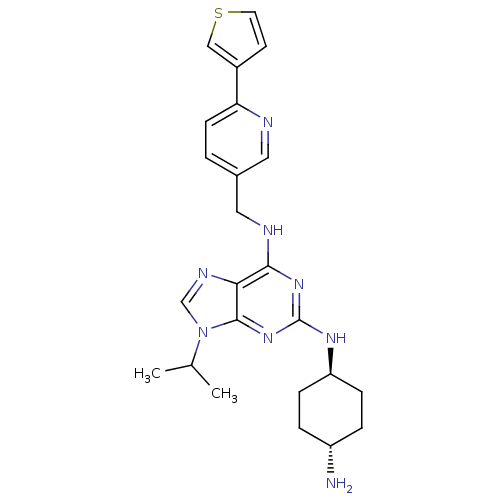
(CHEMBL568080)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-c3ccsc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(.11,-31.85,;1.13,-30.7,;2.64,-31.01,;.64,-29.24,;1.53,-27.99,;.62,-26.75,;-.84,-27.24,;-2.17,-26.48,;-2.18,-24.94,;-3.52,-24.18,;-4.85,-24.96,;-6.18,-24.19,;-7.51,-24.97,;-7.5,-26.51,;-6.15,-27.27,;-4.83,-26.49,;-8.83,-27.29,;-8.98,-28.83,;-10.49,-29.16,;-11.27,-27.83,;-10.24,-26.68,;-3.5,-27.26,;-3.5,-28.8,;-4.83,-29.57,;-4.82,-31.11,;-3.49,-31.87,;-3.49,-33.42,;-4.82,-34.19,;-4.82,-35.73,;-6.16,-33.42,;-6.16,-31.89,;-2.16,-29.56,;-.83,-28.78,)| Show InChI InChI=1S/C24H30N8S/c1-15(2)32-14-28-21-22(27-12-16-3-8-20(26-11-16)17-9-10-33-13-17)30-24(31-23(21)32)29-19-6-4-18(25)5-7-19/h3,8-11,13-15,18-19H,4-7,12,25H2,1-2H3,(H2,27,29,30,31)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378288

(CHEMBL567434)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-c3ccccn3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.26,wD:28.30,(14.81,-30.86,;15.83,-29.71,;17.34,-30.01,;15.34,-28.25,;16.23,-27,;15.32,-25.76,;13.86,-26.25,;12.53,-25.49,;12.52,-23.95,;11.18,-23.19,;9.85,-23.96,;8.52,-23.2,;7.19,-23.98,;7.2,-25.52,;8.55,-26.28,;9.87,-25.5,;5.88,-26.3,;5.89,-27.83,;4.57,-28.61,;3.22,-27.85,;3.21,-26.31,;4.54,-25.53,;11.2,-26.26,;11.2,-27.8,;9.87,-28.58,;9.88,-30.12,;11.21,-30.88,;11.21,-32.43,;9.88,-33.2,;9.88,-34.74,;8.54,-32.43,;8.54,-30.89,;12.54,-28.57,;13.88,-27.78,)| Show InChI InChI=1S/C25H31N9/c1-16(2)34-15-30-22-23(32-25(33-24(22)34)31-19-9-7-18(26)8-10-19)29-14-17-6-11-21(28-13-17)20-5-3-4-12-27-20/h3-6,11-13,15-16,18-19H,7-10,14,26H2,1-2H3,(H2,29,31,32,33)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378290
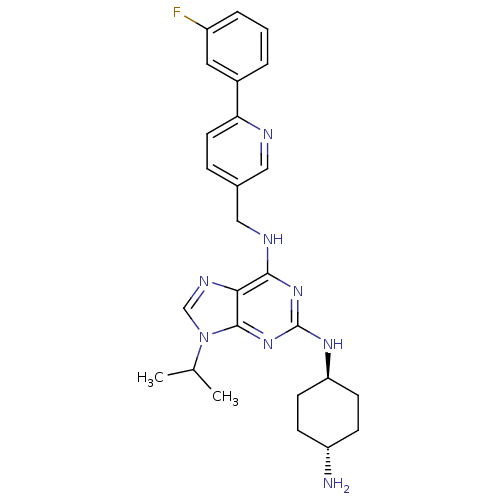
(CHEMBL568310)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-c3cccc(F)c3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:26.27,wD:29.31,(29.87,-.79,;30.89,.36,;32.4,.06,;30.4,1.83,;31.3,3.08,;30.38,4.31,;28.93,3.82,;27.59,4.58,;27.59,6.12,;26.25,6.88,;24.92,6.11,;24.92,4.56,;23.59,3.79,;22.26,4.56,;22.25,6.09,;23.57,6.87,;20.93,3.78,;20.94,2.25,;19.61,1.47,;18.27,2.23,;18.26,3.78,;16.92,4.54,;19.59,4.55,;26.26,3.81,;26.27,2.27,;24.94,1.49,;24.95,-.05,;26.28,-.81,;26.28,-2.35,;24.94,-3.13,;24.94,-4.67,;23.61,-2.36,;23.61,-.82,;27.61,1.5,;28.94,2.29,)| Show InChI InChI=1S/C26H31FN8/c1-16(2)35-15-31-23-24(33-26(34-25(23)35)32-21-9-7-20(28)8-10-21)30-14-17-6-11-22(29-13-17)18-4-3-5-19(27)12-18/h3-6,11-13,15-16,20-21H,7-10,14,28H2,1-2H3,(H2,30,32,33,34)/t20-,21- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378291
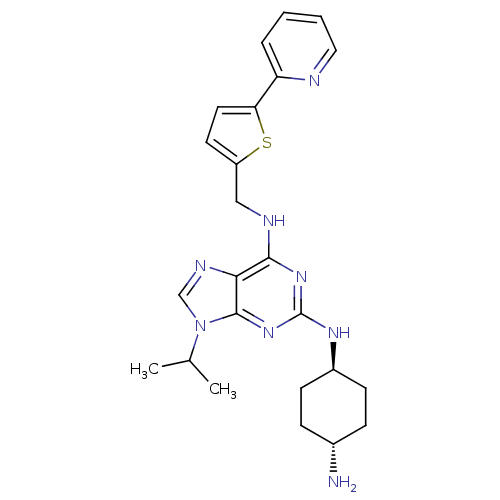
(CHEMBL568302)Show SMILES CC(C)n1cnc2c(NCc3ccc(s3)-c3ccccn3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(16.31,-45.59,;17.34,-44.44,;18.84,-44.74,;16.85,-42.97,;17.74,-41.72,;16.83,-40.49,;15.37,-40.98,;14.04,-40.22,;14.03,-38.68,;12.69,-37.92,;11.36,-38.69,;11.2,-40.23,;9.7,-40.56,;8.92,-39.23,;9.95,-38.08,;7.38,-39.23,;6.62,-37.9,;5.08,-37.9,;4.31,-39.23,;5.09,-40.57,;6.63,-40.56,;12.7,-40.99,;12.71,-42.53,;11.38,-43.31,;11.39,-44.85,;12.72,-45.61,;12.72,-47.15,;11.39,-47.93,;11.39,-49.47,;10.05,-47.16,;10.05,-45.62,;14.05,-43.3,;15.38,-42.51,)| Show InChI InChI=1S/C24H30N8S/c1-15(2)32-14-28-21-22(27-13-18-10-11-20(33-18)19-5-3-4-12-26-19)30-24(31-23(21)32)29-17-8-6-16(25)7-9-17/h3-5,10-12,14-17H,6-9,13,25H2,1-2H3,(H2,27,29,30,31)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378305
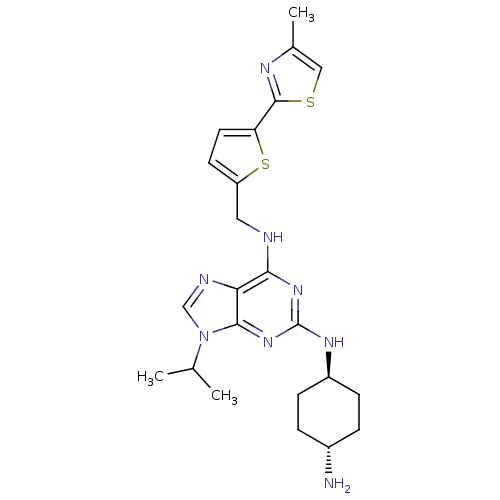
(CHEMBL583417)Show SMILES CC(C)n1cnc2c(NCc3ccc(s3)-c3nc(C)cs3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(1.97,-46.51,;2.99,-45.36,;4.49,-45.66,;2.5,-43.9,;3.39,-42.65,;2.48,-41.41,;1.02,-41.9,;-.31,-41.14,;-.32,-39.6,;-1.66,-38.84,;-2.99,-39.61,;-3.15,-41.15,;-4.65,-41.48,;-5.43,-40.15,;-4.4,-39,;-6.97,-40.15,;-7.87,-38.91,;-9.34,-39.39,;-10.58,-38.49,;-9.33,-40.93,;-7.87,-41.4,;-1.65,-41.91,;-1.64,-43.45,;-2.97,-44.23,;-2.96,-45.77,;-1.63,-46.53,;-1.63,-48.08,;-2.96,-48.85,;-2.96,-50.39,;-4.3,-48.08,;-4.3,-46.54,;-.3,-44.22,;1.03,-43.43,)| Show InChI InChI=1S/C23H30N8S2/c1-13(2)31-12-26-19-20(25-10-17-8-9-18(33-17)22-27-14(3)11-32-22)29-23(30-21(19)31)28-16-6-4-15(24)5-7-16/h8-9,11-13,15-16H,4-7,10,24H2,1-3H3,(H2,25,28,29,30)/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378319

(CHEMBL568303)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-c3cccs3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(30.87,-45.55,;31.89,-44.4,;33.4,-44.7,;31.4,-42.94,;32.29,-41.69,;31.38,-40.45,;29.92,-40.94,;28.59,-40.18,;28.58,-38.64,;27.24,-37.88,;25.91,-38.65,;24.58,-37.89,;23.25,-38.67,;23.26,-40.21,;24.61,-40.97,;25.93,-40.19,;21.93,-40.99,;21.78,-42.52,;20.27,-42.85,;19.49,-41.52,;20.52,-40.37,;27.26,-40.96,;27.26,-42.49,;25.93,-43.27,;25.94,-44.81,;27.27,-45.57,;27.27,-47.12,;25.94,-47.89,;25.94,-49.43,;24.6,-47.12,;24.6,-45.58,;28.6,-43.26,;29.94,-42.47,)| Show InChI InChI=1S/C24H30N8S/c1-15(2)32-14-28-21-22(27-13-16-5-10-19(26-12-16)20-4-3-11-33-20)30-24(31-23(21)32)29-18-8-6-17(25)7-9-18/h3-5,10-12,14-15,17-18H,6-9,13,25H2,1-2H3,(H2,27,29,30,31)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378289
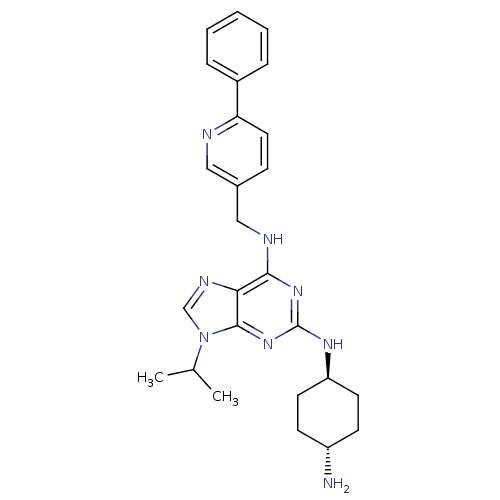
(CHEMBL565705)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-c3ccccc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.26,wD:28.30,(1.05,-1.82,;2.08,-.67,;3.58,-.98,;1.59,.79,;2.48,2.04,;1.57,3.28,;.11,2.79,;-1.22,3.55,;-1.23,5.09,;-2.57,5.85,;-3.9,5.07,;-3.89,3.53,;-5.22,2.76,;-6.56,3.53,;-6.57,5.06,;-5.24,5.84,;-7.89,2.75,;-9.23,3.52,;-10.56,2.74,;-10.55,1.2,;-9.2,.44,;-7.88,1.21,;-2.56,2.77,;-2.55,1.23,;-3.88,.46,;-3.87,-1.08,;-2.54,-1.84,;-2.54,-3.39,;-3.87,-4.16,;-3.87,-5.7,;-5.21,-3.39,;-5.21,-1.86,;-1.21,.47,;.12,1.25,)| Show InChI InChI=1S/C26H32N8/c1-17(2)34-16-30-23-24(32-26(33-25(23)34)31-21-11-9-20(27)10-12-21)29-15-18-8-13-22(28-14-18)19-6-4-3-5-7-19/h3-8,13-14,16-17,20-21H,9-12,15,27H2,1-2H3,(H2,29,31,32,33)/t20-,21- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50003691
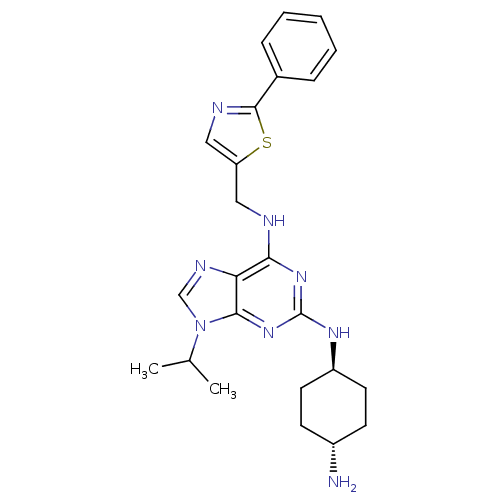
(CHEMBL567624)Show SMILES Cl.CC(C)n1cnc2c(NCc3cnc(s3)-c3ccccc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.25,wD:28.29,(.89,-6.67,;1.17,-16.84,;2.19,-15.69,;3.7,-16,;1.7,-14.23,;2.6,-12.98,;1.68,-11.74,;.23,-12.23,;-1.11,-11.47,;-1.11,-9.93,;-2.45,-9.17,;-3.78,-9.95,;-3.94,-11.49,;-5.44,-11.81,;-6.22,-10.48,;-5.2,-9.33,;-7.76,-10.49,;-8.53,-9.15,;-10.07,-9.15,;-10.83,-10.49,;-10.05,-11.83,;-8.52,-11.82,;-2.44,-12.25,;-2.43,-13.79,;-3.76,-14.56,;-3.75,-16.1,;-2.42,-16.86,;-2.42,-18.41,;-3.76,-19.18,;-3.76,-20.72,;-5.09,-18.41,;-5.09,-16.88,;-1.09,-14.55,;.24,-13.77,)| Show InChI InChI=1S/C24H30N8S.ClH/c1-15(2)32-14-28-20-21(26-12-19-13-27-23(33-19)16-6-4-3-5-7-16)30-24(31-22(20)32)29-18-10-8-17(25)9-11-18;/h3-7,13-15,17-18H,8-12,25H2,1-2H3,(H2,26,29,30,31);1H/t17-,18-; | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50003691

(CHEMBL567624)Show SMILES Cl.CC(C)n1cnc2c(NCc3cnc(s3)-c3ccccc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.25,wD:28.29,(.89,-6.67,;1.17,-16.84,;2.19,-15.69,;3.7,-16,;1.7,-14.23,;2.6,-12.98,;1.68,-11.74,;.23,-12.23,;-1.11,-11.47,;-1.11,-9.93,;-2.45,-9.17,;-3.78,-9.95,;-3.94,-11.49,;-5.44,-11.81,;-6.22,-10.48,;-5.2,-9.33,;-7.76,-10.49,;-8.53,-9.15,;-10.07,-9.15,;-10.83,-10.49,;-10.05,-11.83,;-8.52,-11.82,;-2.44,-12.25,;-2.43,-13.79,;-3.76,-14.56,;-3.75,-16.1,;-2.42,-16.86,;-2.42,-18.41,;-3.76,-19.18,;-3.76,-20.72,;-5.09,-18.41,;-5.09,-16.88,;-1.09,-14.55,;.24,-13.77,)| Show InChI InChI=1S/C24H30N8S.ClH/c1-15(2)32-14-28-20-21(26-12-19-13-27-23(33-19)16-6-4-3-5-7-16)30-24(31-22(20)32)29-18-10-8-17(25)9-11-18;/h3-7,13-15,17-18H,8-12,25H2,1-2H3,(H2,26,29,30,31);1H/t17-,18-; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cdk2/cyclinA |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378292
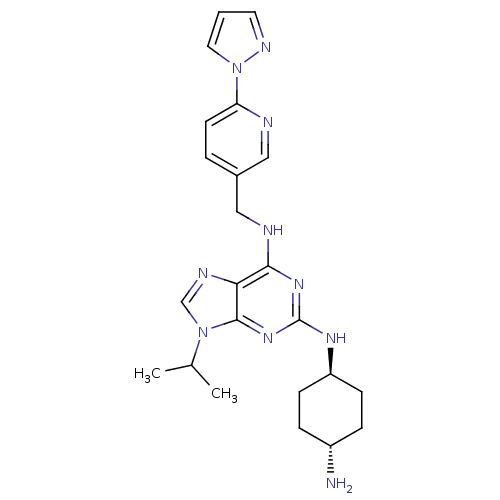
(CHEMBL566106)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-n3cccn3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(46.25,-43.57,;47.27,-42.41,;48.78,-42.72,;46.78,-40.95,;47.68,-39.7,;46.76,-38.47,;45.31,-38.95,;43.97,-38.2,;43.97,-36.66,;42.63,-35.89,;41.3,-36.67,;39.97,-35.91,;38.64,-36.68,;38.65,-38.22,;39.99,-38.99,;41.32,-38.21,;37.31,-39.01,;35.9,-38.39,;34.88,-39.54,;35.66,-40.87,;37.16,-40.54,;42.64,-38.97,;42.65,-40.51,;41.32,-41.29,;41.33,-42.83,;42.66,-43.59,;42.66,-45.13,;41.32,-45.91,;41.32,-47.45,;39.99,-45.13,;39.99,-43.6,;43.99,-41.28,;45.32,-40.49,)| Show InChI InChI=1S/C23H30N10/c1-15(2)32-14-27-20-21(26-13-16-4-9-19(25-12-16)33-11-3-10-28-33)30-23(31-22(20)32)29-18-7-5-17(24)6-8-18/h3-4,9-12,14-15,17-18H,5-8,13,24H2,1-2H3,(H2,26,29,30,31)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378293
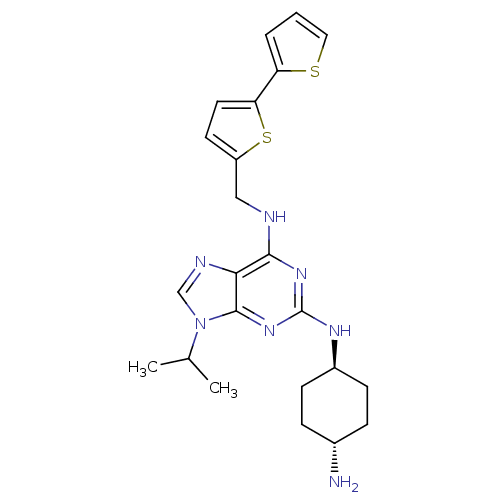
(CHEMBL566550)Show SMILES CC(C)n1cnc2c(NCc3ccc(s3)-c3cccs3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:23.24,wD:26.28,(29.82,-30.94,;30.84,-29.79,;32.35,-30.1,;30.35,-28.33,;31.24,-27.08,;30.33,-25.84,;28.87,-26.33,;27.54,-25.57,;27.53,-24.03,;26.19,-23.27,;24.86,-24.05,;24.71,-25.59,;23.2,-25.91,;22.43,-24.58,;23.45,-23.43,;20.89,-24.59,;19.98,-25.84,;18.52,-25.36,;18.52,-23.82,;19.98,-23.35,;26.21,-26.35,;26.21,-27.89,;24.88,-28.66,;24.89,-30.2,;26.22,-30.96,;26.22,-32.51,;24.89,-33.28,;24.89,-34.82,;23.55,-32.51,;23.55,-30.98,;27.55,-28.65,;28.89,-27.87,)| Show InChI InChI=1S/C23H29N7S2/c1-14(2)30-13-26-20-21(25-12-17-9-10-19(32-17)18-4-3-11-31-18)28-23(29-22(20)30)27-16-7-5-15(24)6-8-16/h3-4,9-11,13-16H,5-8,12,24H2,1-2H3,(H2,25,27,28,29)/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378294
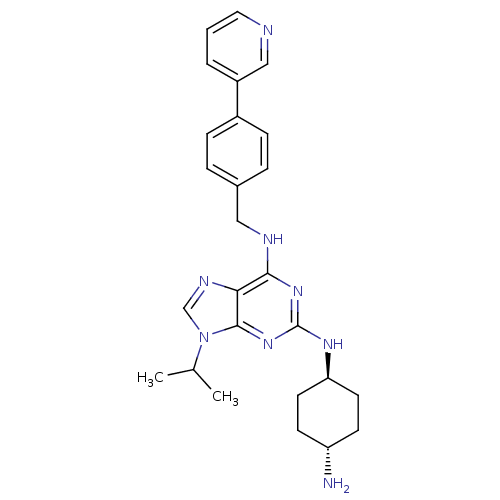
(CHEMBL566972)Show SMILES CC(C)n1cnc2c(NCc3ccc(cc3)-c3cccnc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.26,wD:28.30,(.84,-15.51,;1.87,-14.36,;3.37,-14.67,;1.38,-12.9,;2.27,-11.65,;1.36,-10.41,;-.1,-10.9,;-1.43,-10.14,;-1.44,-8.6,;-2.78,-7.84,;-4.11,-8.62,;-4.1,-10.16,;-5.43,-10.93,;-6.77,-10.16,;-6.78,-8.63,;-5.45,-7.85,;-8.1,-10.94,;-8.09,-12.49,;-9.43,-13.26,;-10.76,-12.49,;-10.76,-10.94,;-9.43,-10.17,;-2.77,-10.92,;-2.76,-12.46,;-4.09,-13.23,;-4.08,-14.77,;-2.75,-15.53,;-2.75,-17.08,;-4.08,-17.85,;-4.08,-19.39,;-5.42,-17.08,;-5.42,-15.55,;-1.42,-13.22,;-.09,-12.44,)| Show InChI InChI=1S/C26H32N8/c1-17(2)34-16-30-23-24(32-26(33-25(23)34)31-22-11-9-21(27)10-12-22)29-14-18-5-7-19(8-6-18)20-4-3-13-28-15-20/h3-8,13,15-17,21-22H,9-12,14,27H2,1-2H3,(H2,29,31,32,33)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50378292

(CHEMBL566106)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-n3cccn3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(46.25,-43.57,;47.27,-42.41,;48.78,-42.72,;46.78,-40.95,;47.68,-39.7,;46.76,-38.47,;45.31,-38.95,;43.97,-38.2,;43.97,-36.66,;42.63,-35.89,;41.3,-36.67,;39.97,-35.91,;38.64,-36.68,;38.65,-38.22,;39.99,-38.99,;41.32,-38.21,;37.31,-39.01,;35.9,-38.39,;34.88,-39.54,;35.66,-40.87,;37.16,-40.54,;42.64,-38.97,;42.65,-40.51,;41.32,-41.29,;41.33,-42.83,;42.66,-43.59,;42.66,-45.13,;41.32,-45.91,;41.32,-47.45,;39.99,-45.13,;39.99,-43.6,;43.99,-41.28,;45.32,-40.49,)| Show InChI InChI=1S/C23H30N10/c1-15(2)32-14-27-20-21(26-13-16-4-9-19(25-12-16)33-11-3-10-28-33)30-23(31-22(20)32)29-18-7-5-17(24)6-8-18/h3-4,9-12,14-15,17-18H,5-8,13,24H2,1-2H3,(H2,26,29,30,31)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cdk2/cyclinA |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50378290

(CHEMBL568310)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-c3cccc(F)c3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:26.27,wD:29.31,(29.87,-.79,;30.89,.36,;32.4,.06,;30.4,1.83,;31.3,3.08,;30.38,4.31,;28.93,3.82,;27.59,4.58,;27.59,6.12,;26.25,6.88,;24.92,6.11,;24.92,4.56,;23.59,3.79,;22.26,4.56,;22.25,6.09,;23.57,6.87,;20.93,3.78,;20.94,2.25,;19.61,1.47,;18.27,2.23,;18.26,3.78,;16.92,4.54,;19.59,4.55,;26.26,3.81,;26.27,2.27,;24.94,1.49,;24.95,-.05,;26.28,-.81,;26.28,-2.35,;24.94,-3.13,;24.94,-4.67,;23.61,-2.36,;23.61,-.82,;27.61,1.5,;28.94,2.29,)| Show InChI InChI=1S/C26H31FN8/c1-16(2)35-15-31-23-24(33-26(34-25(23)35)32-21-9-7-20(28)8-10-21)30-14-17-6-11-22(29-13-17)18-4-3-5-19(27)12-18/h3-6,11-13,15-16,20-21H,7-10,14,28H2,1-2H3,(H2,30,32,33,34)/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cdk2/cyclinA |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378295
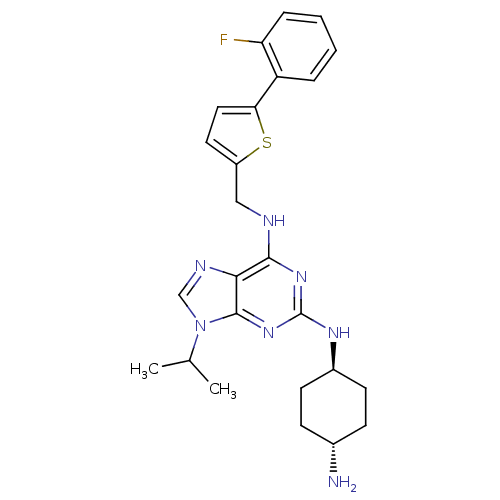
(CHEMBL565490)Show SMILES CC(C)n1cnc2c(NCc3ccc(s3)-c3ccccc3F)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.26,wD:28.30,(16.24,-30.97,;17.26,-29.82,;18.77,-30.13,;16.77,-28.36,;17.66,-27.11,;16.75,-25.88,;15.29,-26.36,;13.96,-25.61,;13.95,-24.07,;12.61,-23.3,;11.28,-24.08,;11.13,-25.62,;9.62,-25.95,;8.85,-24.62,;9.87,-23.47,;7.31,-24.62,;6.54,-25.95,;5.01,-25.96,;4.24,-24.62,;5,-23.29,;6.53,-23.29,;7.3,-21.95,;12.63,-26.38,;12.63,-27.92,;11.3,-28.69,;11.31,-30.23,;12.64,-30.99,;12.64,-32.54,;11.31,-33.31,;11.31,-34.85,;9.97,-32.54,;9.97,-31.01,;13.97,-28.68,;15.31,-27.9,)| Show InChI InChI=1S/C25H30FN7S/c1-15(2)33-14-29-22-23(31-25(32-24(22)33)30-17-9-7-16(27)8-10-17)28-13-18-11-12-21(34-18)19-5-3-4-6-20(19)26/h3-6,11-12,14-17H,7-10,13,27H2,1-2H3,(H2,28,30,31,32)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378296
(CHEMBL568510)Show SMILES CC(C)n1cnc2c(NCc3csc(c3)-c3ccccc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(28.27,-15.16,;29.29,-14.01,;30.8,-14.32,;28.8,-12.55,;29.7,-11.3,;28.78,-10.06,;27.33,-10.55,;25.99,-9.79,;25.99,-8.25,;24.65,-7.49,;23.32,-8.27,;21.91,-7.66,;20.89,-8.81,;21.66,-10.14,;23.17,-9.81,;20.91,-11.47,;19.37,-11.49,;18.62,-12.83,;19.4,-14.16,;20.95,-14.13,;21.7,-12.79,;24.66,-10.57,;24.67,-12.11,;23.34,-12.88,;23.34,-14.42,;24.68,-15.18,;24.68,-16.73,;23.34,-17.5,;23.34,-19.04,;22.01,-16.73,;22,-15.2,;26.01,-12.87,;27.34,-12.09,)| Show InChI InChI=1S/C25H31N7S/c1-16(2)32-15-28-22-23(27-13-17-12-21(33-14-17)18-6-4-3-5-7-18)30-25(31-24(22)32)29-20-10-8-19(26)9-11-20/h3-7,12,14-16,19-20H,8-11,13,26H2,1-2H3,(H2,27,29,30,31)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
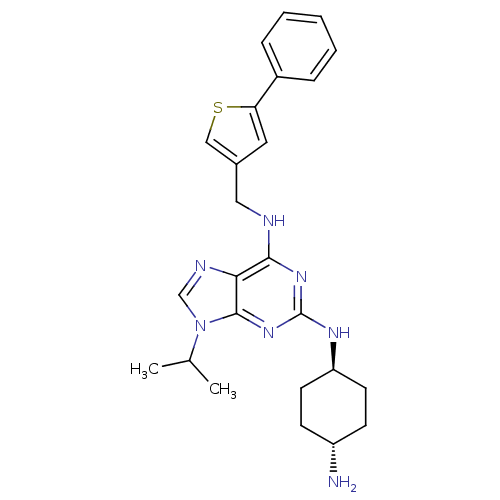
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378297
(CHEMBL566105)Show SMILES CC(C)n1cnc2c(NCc3ccc(cc3)-c3cccs3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(15.61,-15.74,;16.64,-14.59,;18.14,-14.9,;16.15,-13.13,;17.04,-11.88,;16.13,-10.65,;14.67,-11.13,;13.34,-10.38,;13.33,-8.84,;11.99,-8.07,;10.66,-8.85,;10.67,-10.39,;9.34,-11.17,;8,-10.4,;7.99,-8.86,;9.32,-8.09,;6.67,-11.18,;6.52,-12.71,;5.01,-13.04,;4.24,-11.7,;5.26,-10.56,;12,-11.15,;12.01,-12.69,;10.68,-13.47,;10.69,-15.01,;12.02,-15.77,;12.02,-17.31,;10.69,-18.08,;10.69,-19.62,;9.35,-17.31,;9.35,-15.78,;13.35,-13.45,;14.68,-12.67,)| Show InChI InChI=1S/C25H31N7S/c1-16(2)32-15-28-22-23(27-14-17-5-7-18(8-6-17)21-4-3-13-33-21)30-25(31-24(22)32)29-20-11-9-19(26)10-12-20/h3-8,13,15-16,19-20H,9-12,14,26H2,1-2H3,(H2,27,29,30,31)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
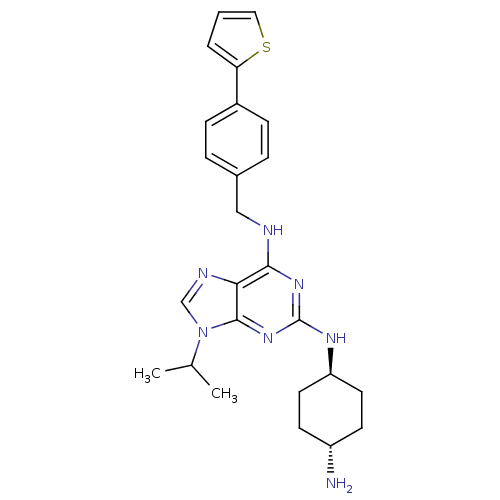
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378298
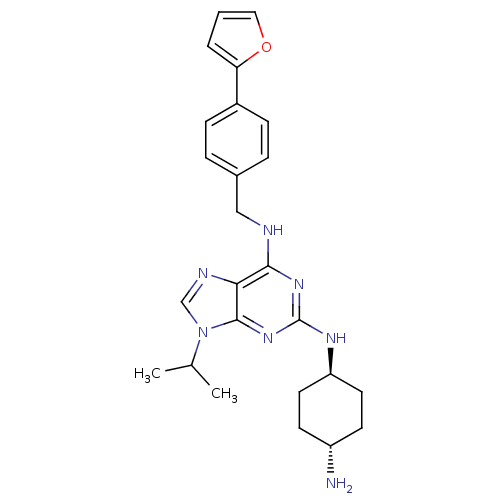
(CHEMBL583421)Show SMILES CC(C)n1cnc2c(NCc3ccc(cc3)-c3ccco3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(15.33,-28.96,;16.35,-27.81,;17.86,-28.11,;15.86,-26.35,;16.75,-25.1,;15.84,-23.86,;14.38,-24.35,;13.05,-23.59,;13.04,-22.05,;11.7,-21.29,;10.37,-22.06,;10.38,-23.61,;9.05,-24.38,;7.71,-23.61,;7.7,-22.08,;9.03,-21.3,;6.38,-24.39,;6.23,-25.92,;4.72,-26.25,;3.95,-24.92,;4.97,-23.77,;11.72,-24.37,;11.72,-25.9,;10.39,-26.68,;10.4,-28.22,;11.73,-28.98,;11.73,-30.53,;10.4,-31.3,;10.4,-32.84,;9.06,-30.53,;9.06,-28.99,;13.06,-26.67,;14.4,-25.88,)| Show InChI InChI=1S/C25H31N7O/c1-16(2)32-15-28-22-23(27-14-17-5-7-18(8-6-17)21-4-3-13-33-21)30-25(31-24(22)32)29-20-11-9-19(26)10-12-20/h3-8,13,15-16,19-20H,9-12,14,26H2,1-2H3,(H2,27,29,30,31)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378299
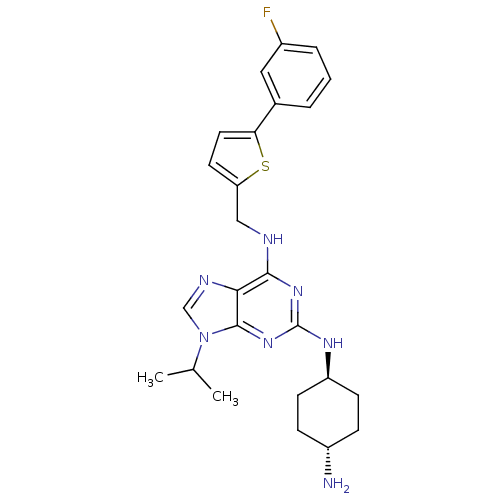
(CHEMBL568079)Show SMILES CC(C)n1cnc2c(NCc3ccc(s3)-c3cccc(F)c3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.26,wD:28.30,(30.5,-30.58,;31.52,-29.42,;33.03,-29.73,;31.03,-27.96,;31.93,-26.71,;31.01,-25.48,;29.56,-25.97,;28.22,-25.21,;28.22,-23.67,;26.88,-22.91,;25.55,-23.68,;25.39,-25.22,;23.89,-25.55,;23.11,-24.22,;24.13,-23.07,;21.57,-24.22,;20.81,-25.56,;19.28,-25.56,;18.5,-24.23,;19.26,-22.89,;18.49,-21.56,;20.8,-22.89,;26.89,-25.98,;26.9,-27.52,;25.57,-28.3,;25.58,-29.84,;26.91,-30.6,;26.91,-32.14,;25.57,-32.92,;25.57,-34.46,;24.24,-32.15,;24.24,-30.61,;28.24,-28.29,;29.57,-27.5,)| Show InChI InChI=1S/C25H30FN7S/c1-15(2)33-14-29-22-23(31-25(32-24(22)33)30-19-8-6-18(27)7-9-19)28-13-20-10-11-21(34-20)16-4-3-5-17(26)12-16/h3-5,10-12,14-15,18-19H,6-9,13,27H2,1-2H3,(H2,28,30,31,32)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50378288

(CHEMBL567434)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-c3ccccn3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.26,wD:28.30,(14.81,-30.86,;15.83,-29.71,;17.34,-30.01,;15.34,-28.25,;16.23,-27,;15.32,-25.76,;13.86,-26.25,;12.53,-25.49,;12.52,-23.95,;11.18,-23.19,;9.85,-23.96,;8.52,-23.2,;7.19,-23.98,;7.2,-25.52,;8.55,-26.28,;9.87,-25.5,;5.88,-26.3,;5.89,-27.83,;4.57,-28.61,;3.22,-27.85,;3.21,-26.31,;4.54,-25.53,;11.2,-26.26,;11.2,-27.8,;9.87,-28.58,;9.88,-30.12,;11.21,-30.88,;11.21,-32.43,;9.88,-33.2,;9.88,-34.74,;8.54,-32.43,;8.54,-30.89,;12.54,-28.57,;13.88,-27.78,)| Show InChI InChI=1S/C25H31N9/c1-16(2)34-15-30-22-23(32-25(33-24(22)34)31-19-9-7-18(26)8-10-19)29-14-17-6-11-21(28-13-17)20-5-3-4-12-27-20/h3-6,11-13,15-16,18-19H,7-10,14,26H2,1-2H3,(H2,29,31,32,33)/t18-,19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cdk2/cyclinA |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50378297

(CHEMBL566105)Show SMILES CC(C)n1cnc2c(NCc3ccc(cc3)-c3cccs3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(15.61,-15.74,;16.64,-14.59,;18.14,-14.9,;16.15,-13.13,;17.04,-11.88,;16.13,-10.65,;14.67,-11.13,;13.34,-10.38,;13.33,-8.84,;11.99,-8.07,;10.66,-8.85,;10.67,-10.39,;9.34,-11.17,;8,-10.4,;7.99,-8.86,;9.32,-8.09,;6.67,-11.18,;6.52,-12.71,;5.01,-13.04,;4.24,-11.7,;5.26,-10.56,;12,-11.15,;12.01,-12.69,;10.68,-13.47,;10.69,-15.01,;12.02,-15.77,;12.02,-17.31,;10.69,-18.08,;10.69,-19.62,;9.35,-17.31,;9.35,-15.78,;13.35,-13.45,;14.68,-12.67,)| Show InChI InChI=1S/C25H31N7S/c1-16(2)32-15-28-22-23(27-14-17-5-7-18(8-6-17)21-4-3-13-33-21)30-25(31-24(22)32)29-20-11-9-19(26)10-12-20/h3-8,13,15-16,19-20H,9-12,14,26H2,1-2H3,(H2,27,29,30,31)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cdk2/cyclinA |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50378289

(CHEMBL565705)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-c3ccccc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.26,wD:28.30,(1.05,-1.82,;2.08,-.67,;3.58,-.98,;1.59,.79,;2.48,2.04,;1.57,3.28,;.11,2.79,;-1.22,3.55,;-1.23,5.09,;-2.57,5.85,;-3.9,5.07,;-3.89,3.53,;-5.22,2.76,;-6.56,3.53,;-6.57,5.06,;-5.24,5.84,;-7.89,2.75,;-9.23,3.52,;-10.56,2.74,;-10.55,1.2,;-9.2,.44,;-7.88,1.21,;-2.56,2.77,;-2.55,1.23,;-3.88,.46,;-3.87,-1.08,;-2.54,-1.84,;-2.54,-3.39,;-3.87,-4.16,;-3.87,-5.7,;-5.21,-3.39,;-5.21,-1.86,;-1.21,.47,;.12,1.25,)| Show InChI InChI=1S/C26H32N8/c1-17(2)34-16-30-23-24(32-26(33-25(23)34)31-21-11-9-20(27)10-12-21)29-15-18-8-13-22(28-14-18)19-6-4-3-5-7-19/h3-8,13-14,16-17,20-21H,9-12,15,27H2,1-2H3,(H2,29,31,32,33)/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cdk2/cyclinA |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378302
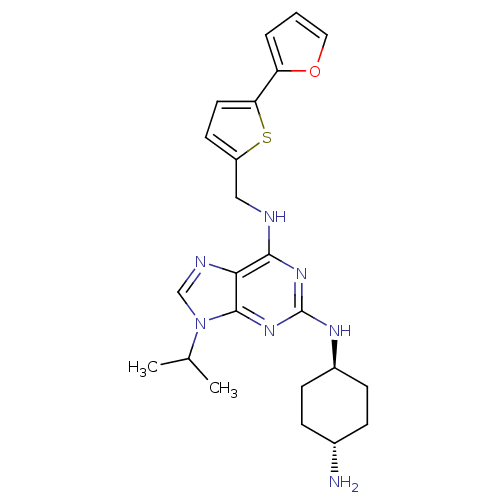
(CHEMBL583615)Show SMILES CC(C)n1cnc2c(NCc3ccc(s3)-c3ccco3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:23.24,wD:26.28,(22.14,-3.25,;23.16,-2.1,;24.67,-2.41,;22.68,-.64,;23.57,.61,;22.65,1.84,;21.2,1.36,;19.86,2.11,;19.86,3.66,;18.52,4.42,;17.19,3.64,;17.03,2.1,;15.53,1.77,;14.75,3.1,;15.77,4.25,;13.21,3.1,;12.31,1.86,;10.84,2.34,;10.84,3.88,;12.31,4.36,;18.53,1.34,;18.54,-.2,;17.21,-.97,;17.22,-2.51,;18.55,-3.28,;18.55,-4.82,;17.21,-5.59,;17.21,-7.13,;15.88,-4.82,;15.88,-3.29,;19.88,-.96,;21.21,-.18,)| Show InChI InChI=1S/C23H29N7OS/c1-14(2)30-13-26-20-21(25-12-17-9-10-19(32-17)18-4-3-11-31-18)28-23(29-22(20)30)27-16-7-5-15(24)6-8-16/h3-4,9-11,13-16H,5-8,12,24H2,1-2H3,(H2,25,27,28,29)/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378301
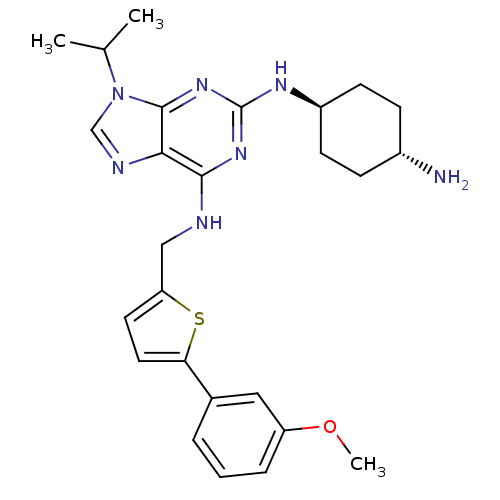
(CHEMBL568311)Show SMILES COc1cccc(c1)-c1ccc(CNc2nc(N[C@H]3CC[C@H](N)CC3)nc3n(cnc23)C(C)C)s1 |r,wU:18.18,wD:21.22,(17.8,-37.74,;17.03,-39.08,;17.81,-40.41,;17.05,-41.74,;17.82,-43.08,;19.35,-43.07,;20.12,-41.74,;19.34,-40.4,;21.66,-41.73,;22.43,-43.06,;23.94,-42.74,;24.09,-41.2,;25.42,-40.42,;26.76,-41.18,;26.77,-42.72,;25.44,-43.5,;25.44,-45.04,;24.11,-45.81,;24.12,-47.35,;25.45,-48.11,;25.45,-49.66,;24.12,-50.43,;24.12,-51.97,;22.78,-49.66,;22.78,-48.13,;26.78,-45.8,;28.12,-45.02,;29.58,-45.48,;30.47,-44.23,;29.56,-42.99,;28.1,-43.48,;30.07,-46.94,;29.05,-48.09,;31.58,-47.25,;22.68,-40.58,)| Show InChI InChI=1S/C26H33N7OS/c1-16(2)33-15-29-23-24(31-26(32-25(23)33)30-19-9-7-18(27)8-10-19)28-14-21-11-12-22(35-21)17-5-4-6-20(13-17)34-3/h4-6,11-13,15-16,18-19H,7-10,14,27H2,1-3H3,(H2,28,30,31,32)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378303
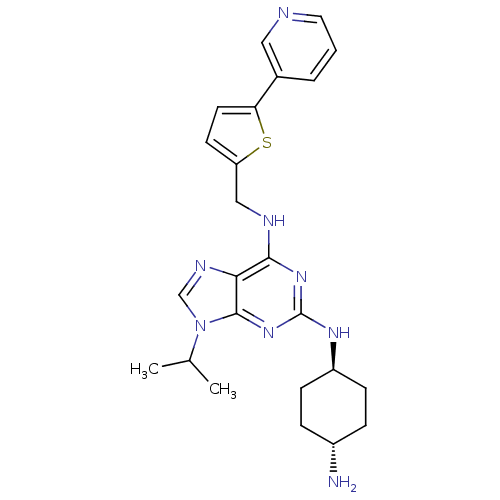
(CHEMBL567208)Show SMILES CC(C)n1cnc2c(NCc3ccc(s3)-c3cccnc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(23.68,-16.97,;24.7,-15.81,;26.21,-16.12,;24.21,-14.35,;25.11,-13.1,;24.19,-11.87,;22.74,-12.35,;21.4,-11.6,;21.39,-10.06,;20.06,-9.29,;18.73,-10.07,;18.57,-11.61,;17.07,-11.94,;16.29,-10.61,;17.31,-9.46,;14.75,-10.61,;13.98,-11.94,;12.46,-11.95,;11.68,-10.61,;12.44,-9.28,;13.98,-9.28,;20.07,-12.37,;20.08,-13.91,;18.75,-14.69,;18.75,-16.23,;20.08,-16.99,;20.09,-18.53,;18.75,-19.31,;18.75,-20.85,;17.42,-18.53,;17.41,-17,;21.42,-14.68,;22.75,-13.89,)| Show InChI InChI=1S/C24H30N8S/c1-15(2)32-14-28-21-22(27-13-19-9-10-20(33-19)16-4-3-11-26-12-16)30-24(31-23(21)32)29-18-7-5-17(25)6-8-18/h3-4,9-12,14-15,17-18H,5-8,13,25H2,1-2H3,(H2,27,29,30,31)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50378304

(CHEMBL568080)Show SMILES CC(C)n1cnc2c(NCc3ccc(nc3)-c3ccsc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(.11,-31.85,;1.13,-30.7,;2.64,-31.01,;.64,-29.24,;1.53,-27.99,;.62,-26.75,;-.84,-27.24,;-2.17,-26.48,;-2.18,-24.94,;-3.52,-24.18,;-4.85,-24.96,;-6.18,-24.19,;-7.51,-24.97,;-7.5,-26.51,;-6.15,-27.27,;-4.83,-26.49,;-8.83,-27.29,;-8.98,-28.83,;-10.49,-29.16,;-11.27,-27.83,;-10.24,-26.68,;-3.5,-27.26,;-3.5,-28.8,;-4.83,-29.57,;-4.82,-31.11,;-3.49,-31.87,;-3.49,-33.42,;-4.82,-34.19,;-4.82,-35.73,;-6.16,-33.42,;-6.16,-31.89,;-2.16,-29.56,;-.83,-28.78,)| Show InChI InChI=1S/C24H30N8S/c1-15(2)32-14-28-21-22(27-12-16-3-8-20(26-11-16)17-9-10-33-13-17)30-24(31-23(21)32)29-19-6-4-18(25)5-7-19/h3,8-11,13-15,18-19H,4-7,12,25H2,1-2H3,(H2,27,29,30,31)/t18-,19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cdk2/cyclinA |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50378305

(CHEMBL583417)Show SMILES CC(C)n1cnc2c(NCc3ccc(s3)-c3nc(C)cs3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:24.25,wD:27.29,(1.97,-46.51,;2.99,-45.36,;4.49,-45.66,;2.5,-43.9,;3.39,-42.65,;2.48,-41.41,;1.02,-41.9,;-.31,-41.14,;-.32,-39.6,;-1.66,-38.84,;-2.99,-39.61,;-3.15,-41.15,;-4.65,-41.48,;-5.43,-40.15,;-4.4,-39,;-6.97,-40.15,;-7.87,-38.91,;-9.34,-39.39,;-10.58,-38.49,;-9.33,-40.93,;-7.87,-41.4,;-1.65,-41.91,;-1.64,-43.45,;-2.97,-44.23,;-2.96,-45.77,;-1.63,-46.53,;-1.63,-48.08,;-2.96,-48.85,;-2.96,-50.39,;-4.3,-48.08,;-4.3,-46.54,;-.3,-44.22,;1.03,-43.43,)| Show InChI InChI=1S/C23H30N8S2/c1-13(2)31-12-26-19-20(25-10-17-8-9-18(33-17)22-27-14(3)11-32-22)29-23(30-21(19)31)28-16-6-4-15(24)5-7-16/h8-9,11-13,15-16H,4-7,10,24H2,1-3H3,(H2,25,28,29,30)/t15-,16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cdk2/cyclinA |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50378212
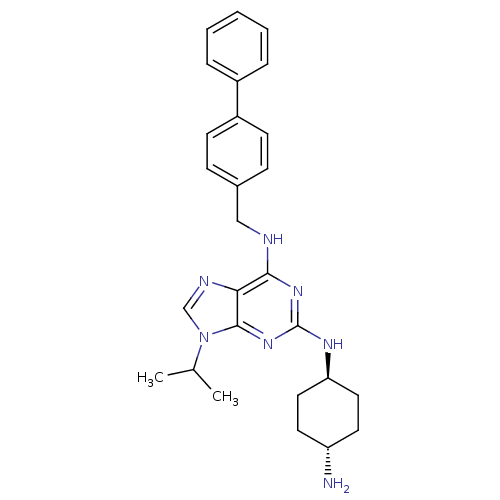
(CHEMBL575450)Show SMILES CC(C)n1cnc2c(NCc3ccc(cc3)-c3ccccc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |r,wU:25.26,wD:28.30,(15.79,-2.71,;16.81,-1.56,;18.32,-1.86,;16.32,-.1,;17.21,1.15,;16.3,2.39,;14.84,1.9,;13.51,2.66,;13.5,4.2,;12.16,4.96,;10.83,4.19,;10.84,2.64,;9.51,1.87,;8.17,2.64,;8.16,4.17,;9.49,4.95,;6.85,1.87,;5.51,2.63,;4.18,1.86,;4.18,.31,;5.51,-.46,;6.85,.32,;12.18,1.88,;12.18,.35,;10.85,-.43,;10.86,-1.97,;12.19,-2.73,;12.19,-4.28,;10.86,-5.05,;10.86,-6.59,;9.52,-4.28,;9.52,-2.74,;13.52,-.42,;14.85,.37,)| Show InChI InChI=1S/C27H33N7/c1-18(2)34-17-30-24-25(32-27(33-26(24)34)31-23-14-12-22(28)13-15-23)29-16-19-8-10-21(11-9-19)20-6-4-3-5-7-20/h3-11,17-18,22-23H,12-16,28H2,1-2H3,(H2,29,31,32,33)/t22-,23- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of Cdk2/cyclinE |
Bioorg Med Chem Lett 19: 6613-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.011
BindingDB Entry DOI: 10.7270/Q2M32WPQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data