

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262340 (11-Amino-3,3-dimethyl-12-p-tolyl-3,4,5,7,8,9,10,12...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262341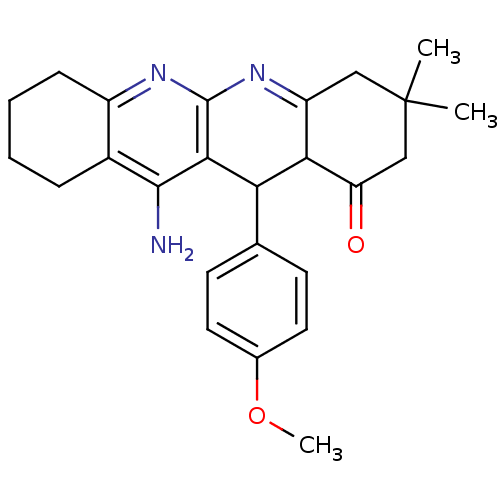 (11-Amino-12-(4-methoxy-phenyl)-3,3-dimethyl-3,4,5,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262282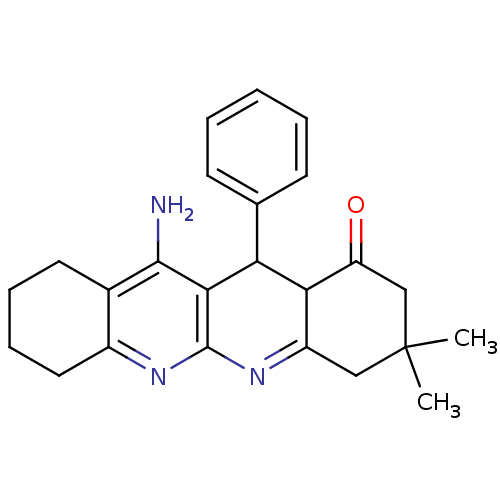 (11-Amino-3,3-dimethyl-12-phenyl-3,4,5,7,8,9,10,12-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 61.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262339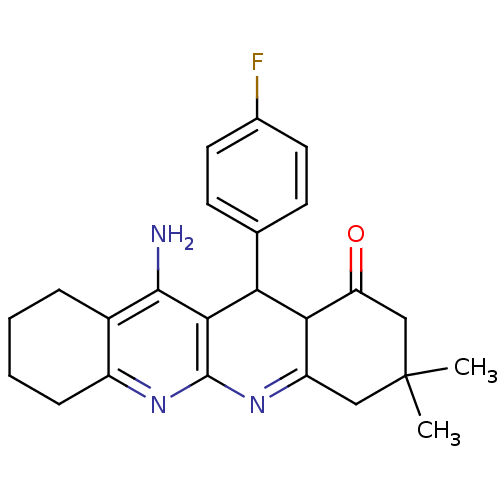 (11-Amino-12-(4-fluoro-phenyl)-3,3-dimethyl-3,4,5,7...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262342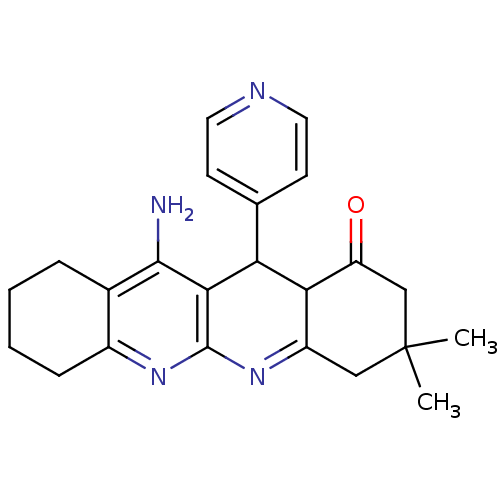 (11-Amino-3,3-dimethyl-12-pyridin-4-yl-3,4,5,7,8,9,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50553103 (CHEMBL4784866) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of kinase tracer 236 from human N-terminal His-tagged MELK (1 to 337 residues) expressed in Escherichia coli BL21 pLysS by microscale th... | Citation and Details Article DOI: 10.1039/c9md00519f BindingDB Entry DOI: 10.7270/Q2TQ655W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM31904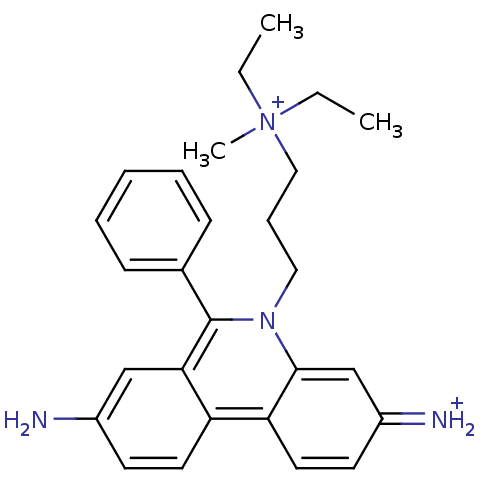 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50369748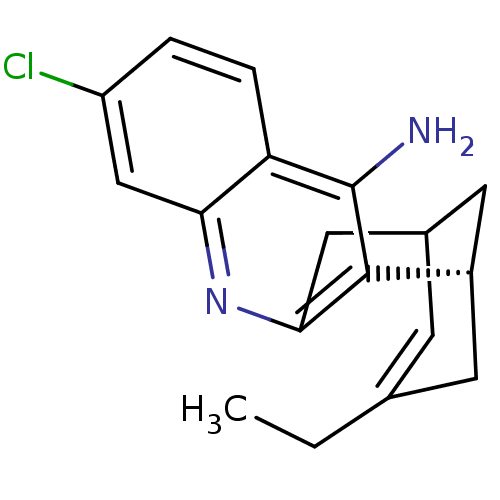 (CHEMBL208599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50369748 (CHEMBL208599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592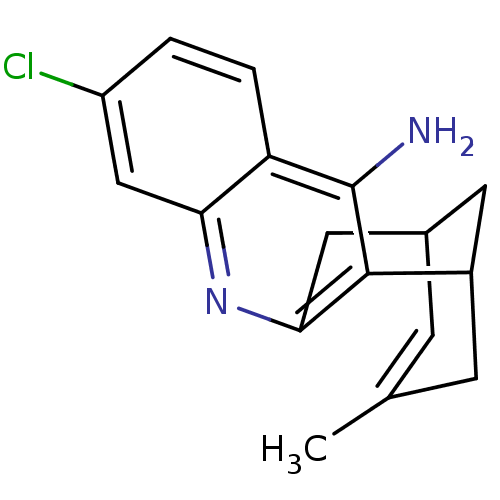 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094626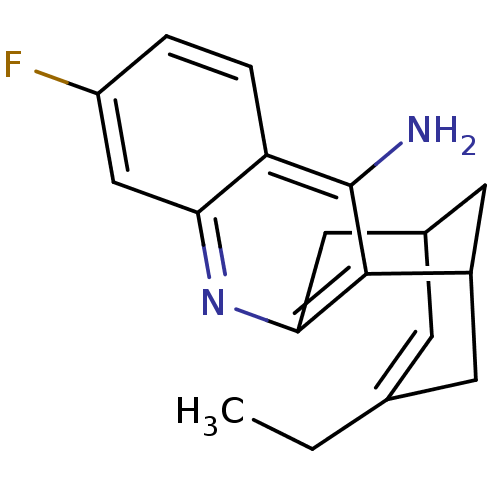 ((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369748 (CHEMBL208599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369748 (CHEMBL208599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50094630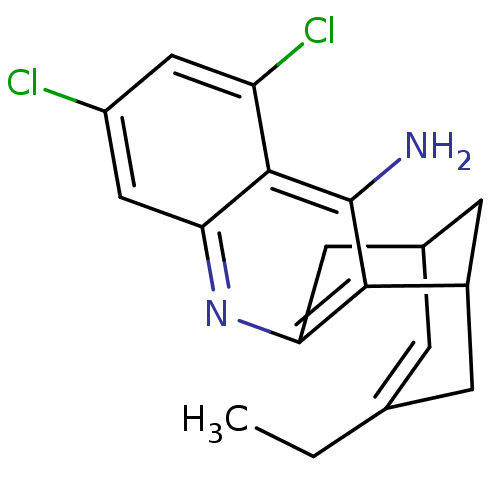 (5,7-dichloro-15-ethyl-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50094631 (15-ethyl-5,7-difluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094626 ((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 0 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50094626 ((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50094626 ((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50094633 (5,7-difluoro-15-methyl-10-azatetracyclo[11.3.1.02,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094633 (5,7-difluoro-15-methyl-10-azatetracyclo[11.3.1.02,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094631 (15-ethyl-5,7-difluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50369748 (CHEMBL208599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50369748 (CHEMBL208599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 0 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 0 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094628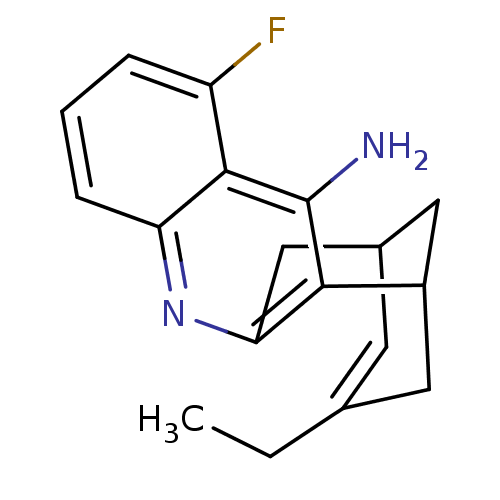 (15-ethyl-5-fluoro-10-azatetracyclo[11.3.1.02,11.04...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 0 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50369748 (CHEMBL208599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 0 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50369748 (CHEMBL208599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 0 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094628 (15-ethyl-5-fluoro-10-azatetracyclo[11.3.1.02,11.04...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094625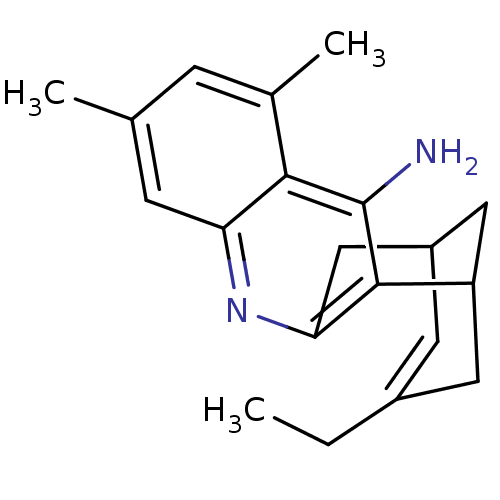 (15-ethyl-5,7-dimethyl-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079861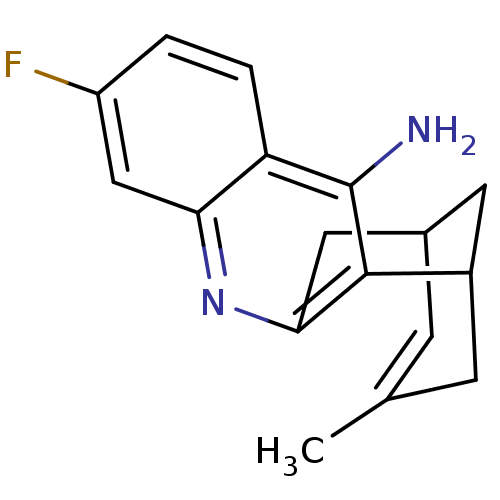 ((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50094625 (15-ethyl-5,7-dimethyl-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.96 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM20928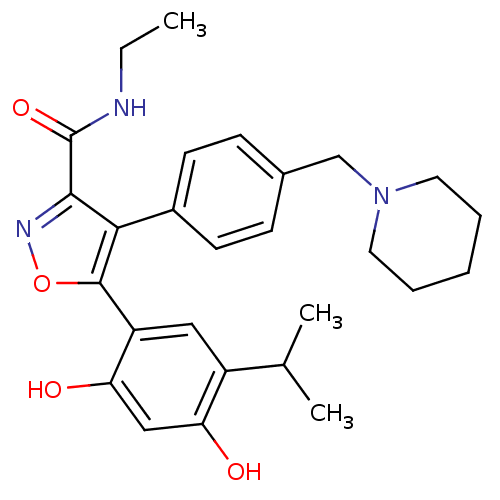 (5-[2,4-dihydroxy-5-(propan-2-yl)phenyl]-N-ethyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | 40 | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The assay is based upon displacement of a fluorescently labeled molecule, which binds specifically to the ATP-binding site of full-length human Hsp90... | J Med Chem 51: 196-218 (2008) Article DOI: 10.1021/jm701018h BindingDB Entry DOI: 10.7270/Q2JW8C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM20927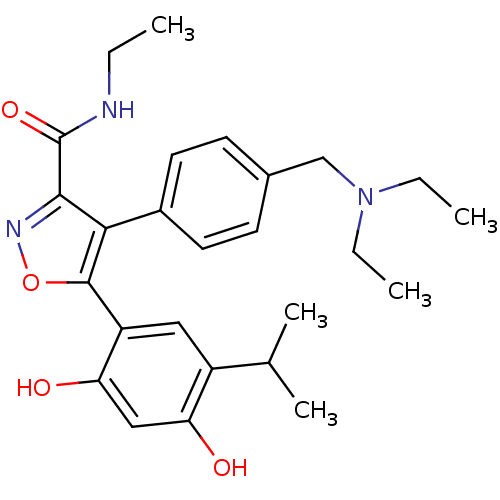 (4-{4-[(diethylamino)methyl]phenyl}-5-[2,4-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | 6 | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The assay is based upon displacement of a fluorescently labeled molecule, which binds specifically to the ATP-binding site of full-length human Hsp90... | J Med Chem 51: 196-218 (2008) Article DOI: 10.1021/jm701018h BindingDB Entry DOI: 10.7270/Q2JW8C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094626 ((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094626 ((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094626 ((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM20924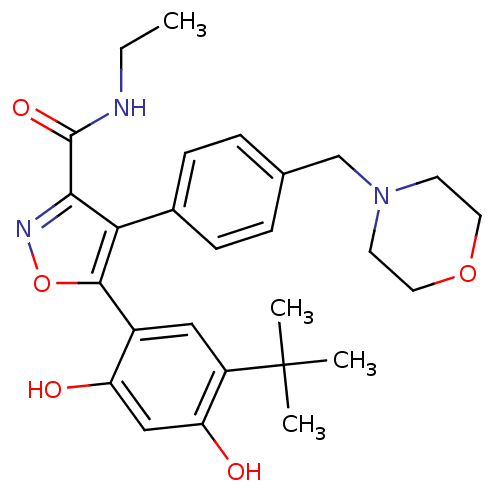 (5-(5-tert-butyl-2,4-dihydroxyphenyl)-N-ethyl-4-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | 70 | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The assay is based upon displacement of a fluorescently labeled molecule, which binds specifically to the ATP-binding site of full-length human Hsp90... | J Med Chem 51: 196-218 (2008) Article DOI: 10.1021/jm701018h BindingDB Entry DOI: 10.7270/Q2JW8C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50079861 ((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes | J Med Chem 42: 3227-42 (1999) Article DOI: 10.1021/jm980620z BindingDB Entry DOI: 10.7270/Q2H70GH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50079861 ((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes | J Med Chem 42: 3227-42 (1999) Article DOI: 10.1021/jm980620z BindingDB Entry DOI: 10.7270/Q2H70GH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50079861 ((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50079861 ((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes | J Med Chem 42: 3227-42 (1999) Article DOI: 10.1021/jm980620z BindingDB Entry DOI: 10.7270/Q2H70GH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 452 total ) | Next | Last >> |