 Found 1747 hits with Last Name = 'bass' and Initial = 'a'
Found 1747 hits with Last Name = 'bass' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548
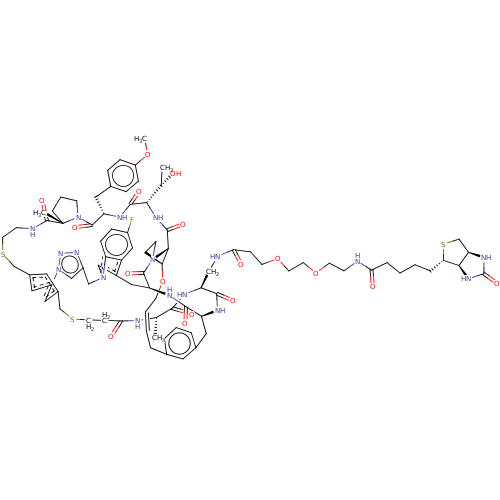
(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547
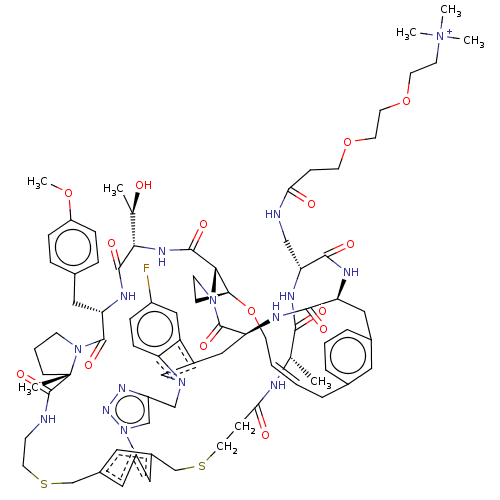
(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546
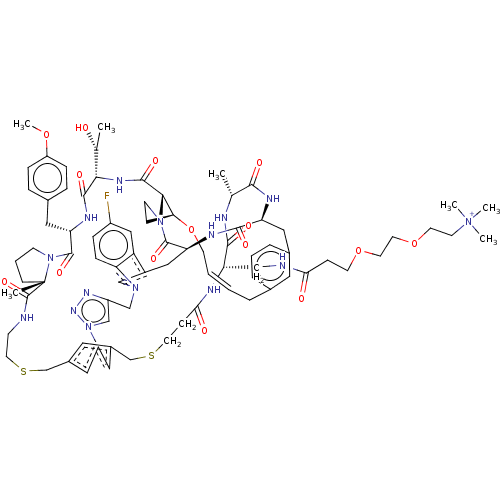
(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545
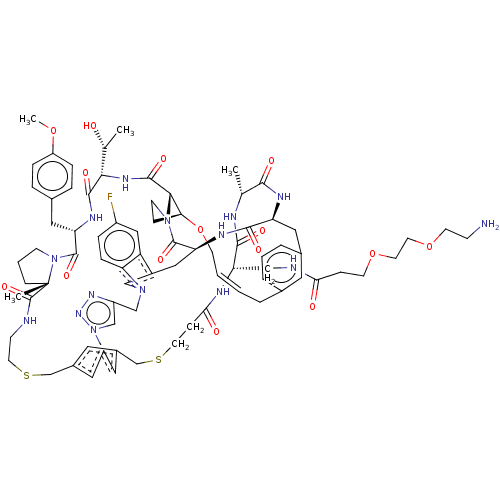
(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544
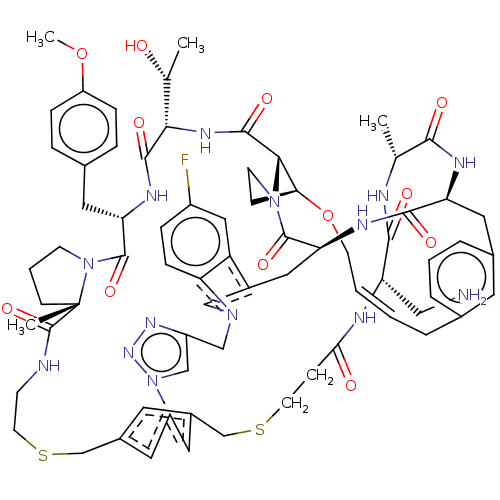
(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581549
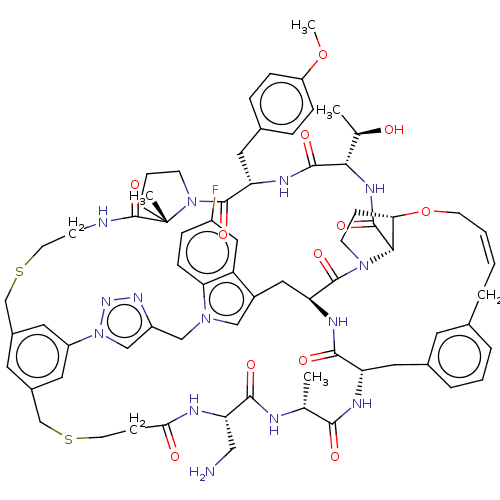
(CHEMBL5082483)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C/CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,c:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545

(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
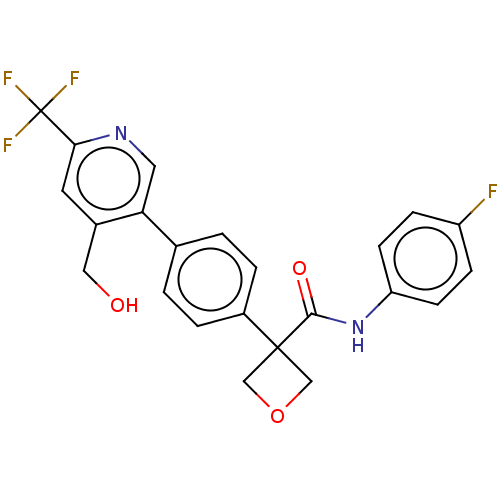
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581542
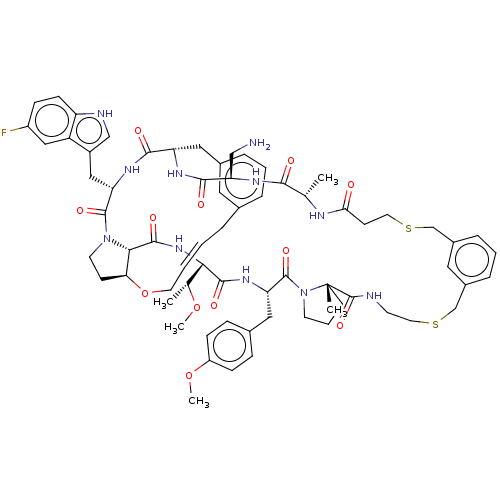
(CHEMBL5081587)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@@H](CN)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581542

(CHEMBL5081587)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@@H](CN)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581540
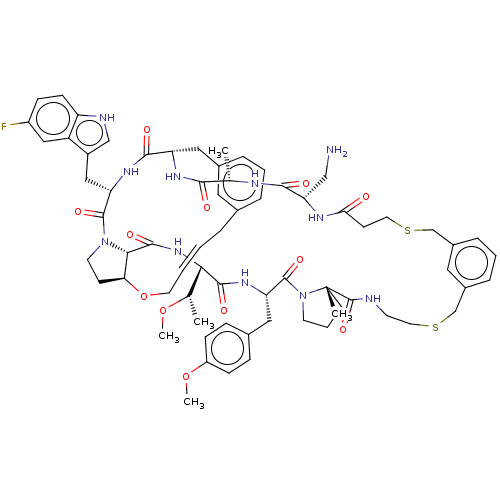
(CHEMBL5087487)Show SMILES CO[C@@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@H](C)NC(=O)[C@H](CN)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581537
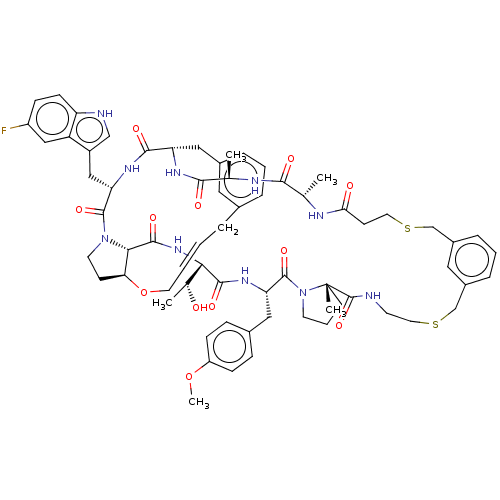
(CHEMBL5086286)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@H](Cc3c[nH]c5ccc(F)cc35)NC(=O)[C@H](Cc3cccc(C\C=C\CO4)c3)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc3cccc(CSCCNC(=O)[C@]4(C)CCCN4C2=O)c3)[C@@H](C)O)cc1 |r,t:48| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581535
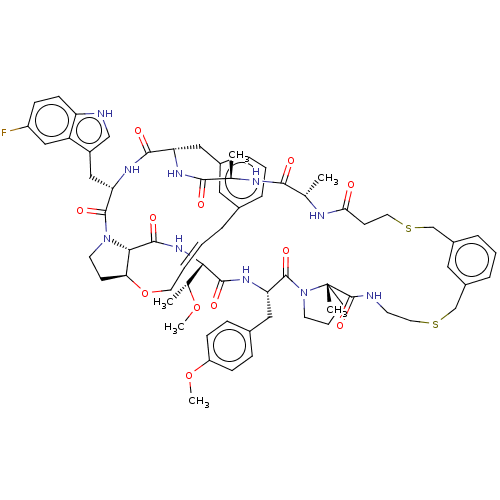
(CHEMBL5091040)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581537

(CHEMBL5086286)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@H](Cc3c[nH]c5ccc(F)cc35)NC(=O)[C@H](Cc3cccc(C\C=C\CO4)c3)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc3cccc(CSCCNC(=O)[C@]4(C)CCCN4C2=O)c3)[C@@H](C)O)cc1 |r,t:48| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581540

(CHEMBL5087487)Show SMILES CO[C@@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@H](C)NC(=O)[C@H](CN)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581535

(CHEMBL5091040)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581543
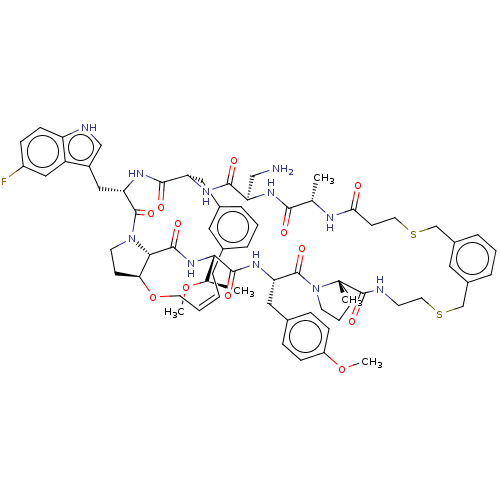
(CHEMBL5094648)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C/CO3)c2)NC(=O)[C@@H](CN)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,c:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50554754
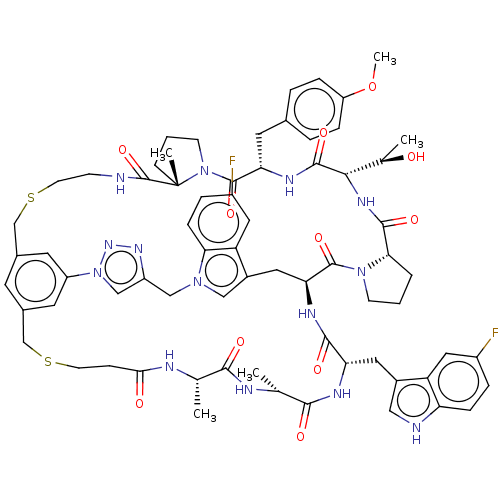
(CHEMBL4790764)Show SMILES [H][C@]1(NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2Cc3cn(Cc4cn(nn4)-c4cc(CSCCNC(=O)[C@]5(C)CCCN5C(=O)[C@H](Cc5ccc(OC)cc5)NC1=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc1c[nH]c5ccc(F)cc15)C(=O)N2)c4)c1ccc(F)cc31)[C@@H](C)O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581539
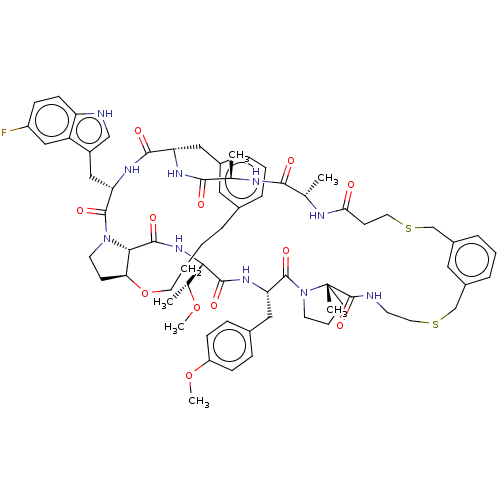
(CHEMBL5084586)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(CCCCO3)c2)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581543

(CHEMBL5094648)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C/CO3)c2)NC(=O)[C@@H](CN)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,c:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581541
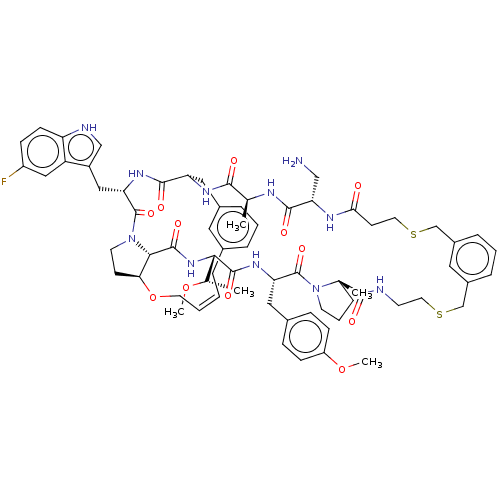
(CHEMBL5078356)Show SMILES CO[C@@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C/CO3)c2)NC(=O)[C@H](C)NC(=O)[C@H](CN)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,c:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581538

(CHEMBL5081995)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@H](Cc3c[nH]c5ccc(F)cc35)NC(=O)[C@H](Cc3cccc(C\C=C/CO4)c3)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc3cccc(CSCCNC(=O)[C@]4(C)CCCN4C2=O)c3)[C@@H](C)O)cc1 |r,c:48| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581536
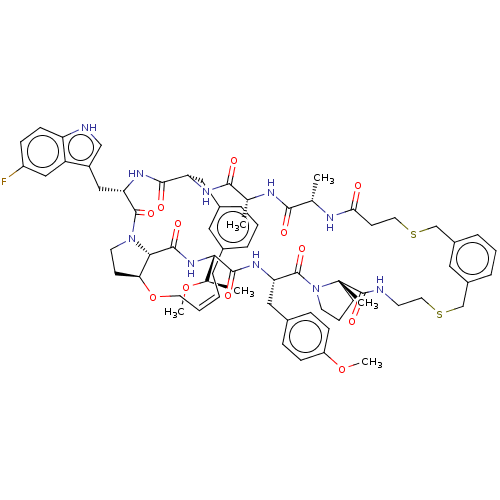
(CHEMBL5081260)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C/CO3)c2)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,c:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50531739
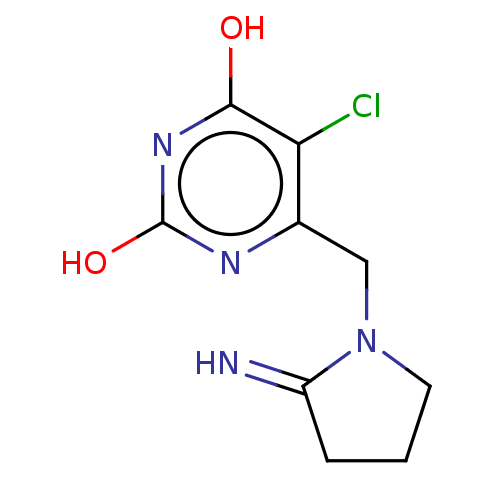
(MA-1 | TPI (freebase) | Tipiracil)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa
Curated by ChEMBL
| Assay Description
Competitive inhibition of thymidine phosphorylase (unknown origin) |
J Med Chem 62: 1231-1245 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01305
BindingDB Entry DOI: 10.7270/Q2FT8QHC |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM586332
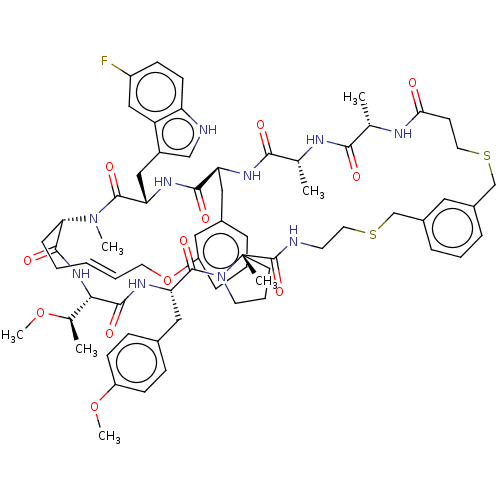
(US11530244, Compound 503)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2CC\C=C\COc3cccc(C[C@H](NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc4cccc(CSCCNC(=O)[C@]5(C)CCCN5C(=O)[C@H](Cc5ccc(OC)cc5)NC1=O)c4)C(=O)N[C@@H](Cc1c[nH]c4ccc(F)cc14)C(=O)N2C)c3 |r,t:11| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM586331
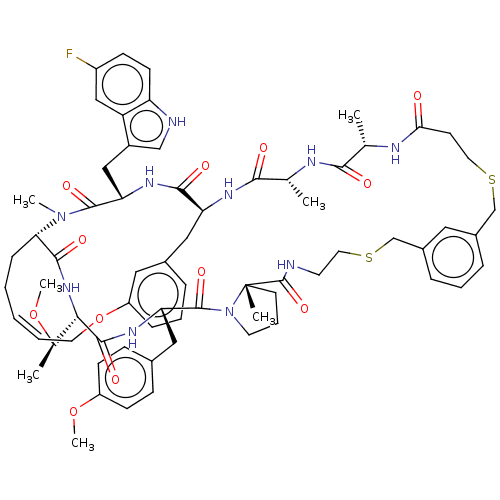
(US11530244, Compound 501)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2CC\C=C/COc3cccc(C[C@H](NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc4cccc(CSCCNC(=O)[C@]5(C)CCCN5C(=O)[C@H](Cc5ccc(OC)cc5)NC1=O)c4)C(=O)N[C@@H](Cc1c[nH]c4ccc(F)cc14)C(=O)N2C)c3 |r,c:11| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50552595
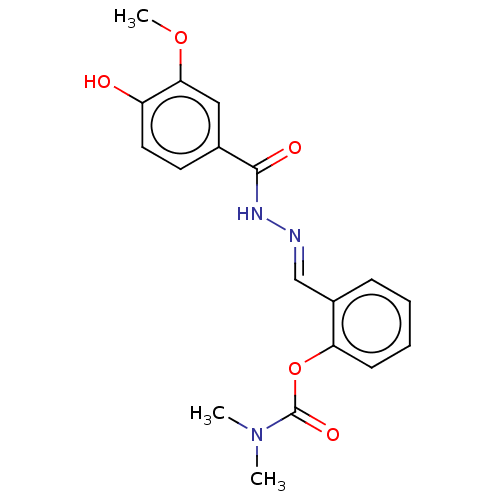
(CHEMBL4761984)Show SMILES COc1cc(ccc1O)C(=O)N\N=C\c1ccccc1OC(=O)N(C)C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Non-Competitive inhibition of equine serum BuChE using varying concentrations of butyrylthiocholine iodide as substrate by Lineweaver-burk plot analy... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115991
BindingDB Entry DOI: 10.7270/Q29W0K3R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075813
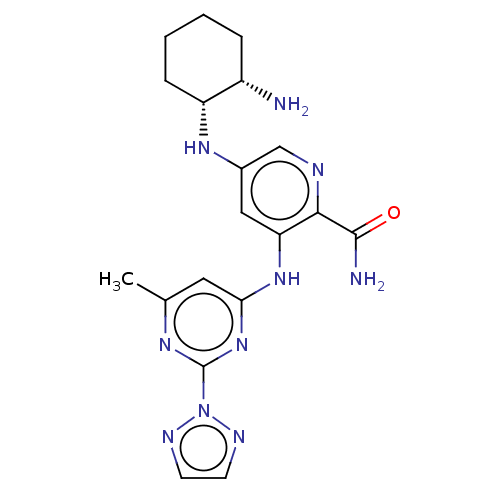
(CHEMBL3415598)Show SMILES Cc1cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)nc(n1)-n1nccn1 |r| Show InChI InChI=1S/C19H24N10O/c1-11-8-16(28-19(25-11)29-23-6-7-24-29)27-15-9-12(10-22-17(15)18(21)30)26-14-5-3-2-4-13(14)20/h6-10,13-14,26H,2-5,20H2,1H3,(H2,21,30)(H,25,27,28)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075735
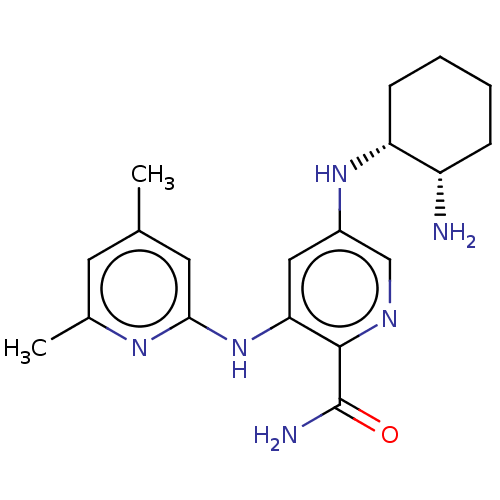
(CHEMBL3415583)Show SMILES Cc1cc(C)nc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)c1 |r| Show InChI InChI=1S/C19H26N6O/c1-11-7-12(2)23-17(8-11)25-16-9-13(10-22-18(16)19(21)26)24-15-6-4-3-5-14(15)20/h7-10,14-15,24H,3-6,20H2,1-2H3,(H2,21,26)(H,23,25)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021
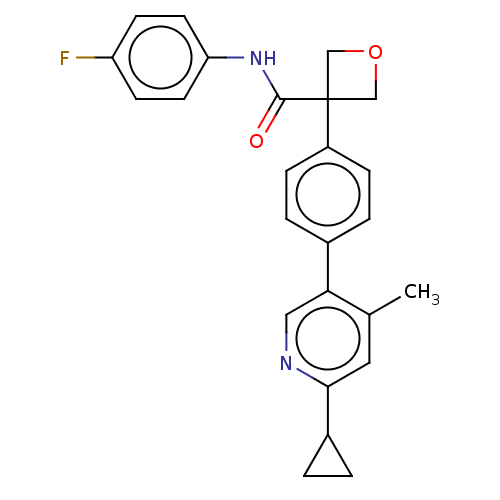
(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
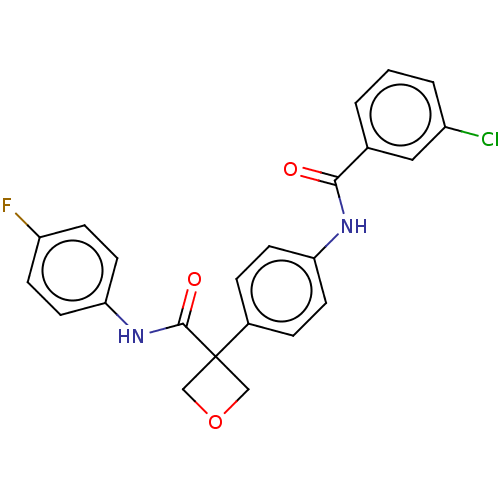
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112904
BindingDB Entry DOI: 10.7270/Q2XP791Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075740

(CHEMBL3415589)Show SMILES Cc1cc(C)nc(Nc2cc(N[C@@H]3CS(=O)(=O)CC[C@@H]3N)cnc2C(N)=O)c1 |r| Show InChI InChI=1S/C18H24N6O3S/c1-10-5-11(2)22-16(6-10)24-14-7-12(8-21-17(14)18(20)25)23-15-9-28(26,27)4-3-13(15)19/h5-8,13,15,23H,3-4,9,19H2,1-2H3,(H2,20,25)(H,22,24)/t13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075922
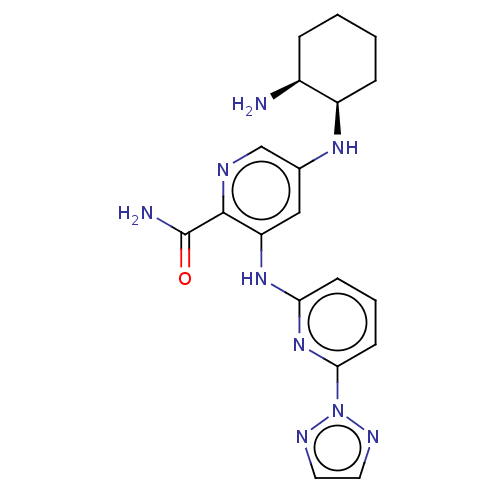
(CHEMBL3415606)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(Nc2cccc(n2)-n2nccn2)c1 |r| Show InChI InChI=1S/C19H23N9O/c20-13-4-1-2-5-14(13)25-12-10-15(18(19(21)29)22-11-12)26-16-6-3-7-17(27-16)28-23-8-9-24-28/h3,6-11,13-14,25H,1-2,4-5,20H2,(H2,21,29)(H,26,27)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075744
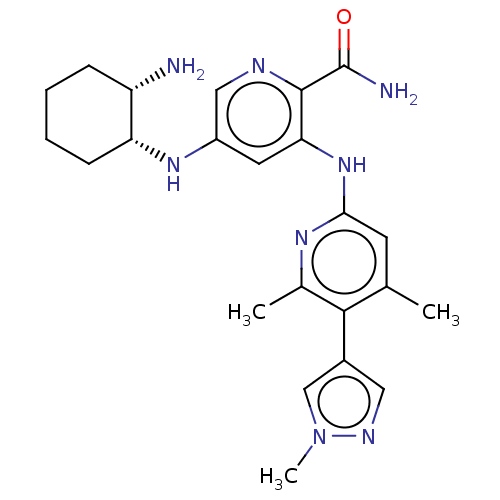
(CHEMBL3415594)Show SMILES Cc1cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)nc(C)c1-c1cnn(C)c1 |r| Show InChI InChI=1S/C23H30N8O/c1-13-8-20(28-14(2)21(13)15-10-27-31(3)12-15)30-19-9-16(11-26-22(19)23(25)32)29-18-7-5-4-6-17(18)24/h8-12,17-18,29H,4-7,24H2,1-3H3,(H2,25,32)(H,28,30)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075747

(CHEMBL3415597)Show SMILES Cc1nc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)cc(OCC(C)(C)O)n1 |r| Show InChI InChI=1S/C21H31N7O3/c1-12-25-17(9-18(26-12)31-11-21(2,3)30)28-16-8-13(10-24-19(16)20(23)29)27-15-7-5-4-6-14(15)22/h8-10,14-15,27,30H,4-7,11,22H2,1-3H3,(H2,23,29)(H,25,26,28)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075819

(CHEMBL3415604)Show SMILES Cc1cc(CCC(C)(C)O)cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)n1 |r| Show InChI InChI=1S/C23H34N6O2/c1-14-10-15(8-9-23(2,3)31)11-20(27-14)29-19-12-16(13-26-21(19)22(25)30)28-18-7-5-4-6-17(18)24/h10-13,17-18,28,31H,4-9,24H2,1-3H3,(H2,25,30)(H,27,29)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075742
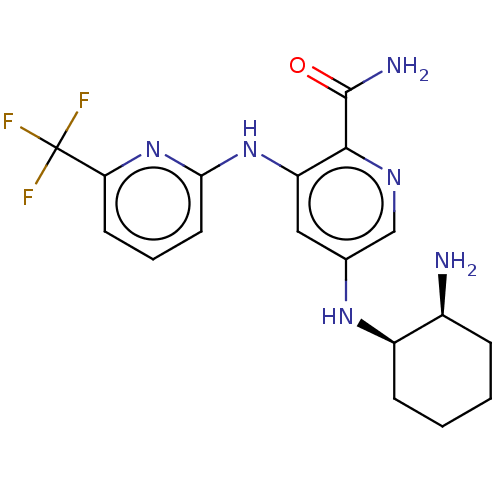
(CHEMBL3415592)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(Nc2cccc(n2)C(F)(F)F)c1 |r| Show InChI InChI=1S/C18H21F3N6O/c19-18(20,21)14-6-3-7-15(27-14)26-13-8-10(9-24-16(13)17(23)28)25-12-5-2-1-4-11(12)22/h3,6-9,11-12,25H,1-2,4-5,22H2,(H2,23,28)(H,26,27)/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075738
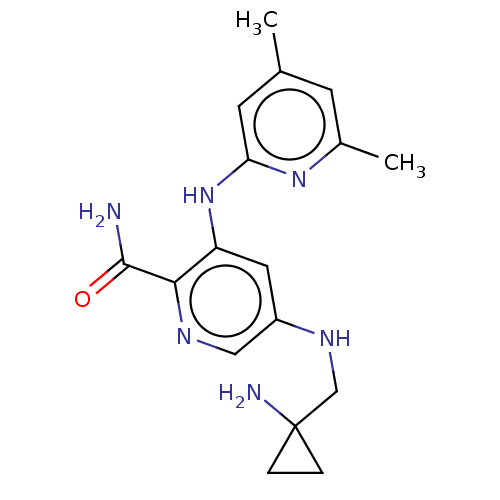
(CHEMBL3415587)Show InChI InChI=1S/C17H22N6O/c1-10-5-11(2)22-14(6-10)23-13-7-12(8-20-15(13)16(18)24)21-9-17(19)3-4-17/h5-8,21H,3-4,9,19H2,1-2H3,(H2,18,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075732
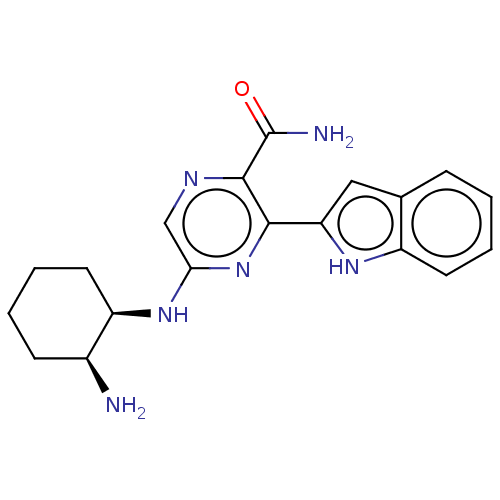
(CHEMBL3414584 | US9775839, 2.1)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(n1)-c1cc2ccccc2[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data