

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosinase (Homo sapiens (Human)) | BDBM50205807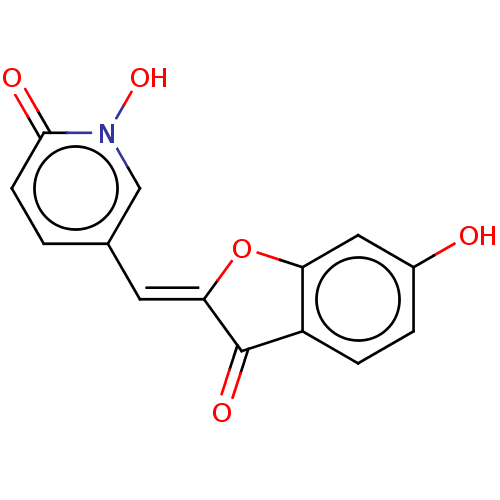 (CHEMBL3978212) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Grenoble-Alpes/CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells assessed as diphenolase activity using L-DOPA as substrate by ... | ACS Med Chem Lett 8: 55-60 (2017) Article DOI: 10.1021/acsmedchemlett.6b00369 BindingDB Entry DOI: 10.7270/Q24F1SQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205815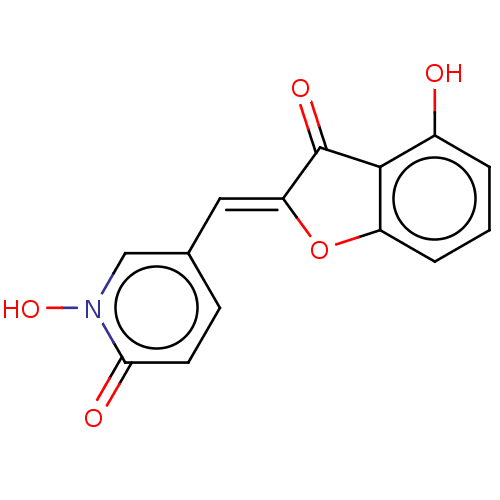 (CHEMBL3907670) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Grenoble-Alpes/CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells assessed as diphenolase activity using L-DOPA as substrate by ... | ACS Med Chem Lett 8: 55-60 (2017) Article DOI: 10.1021/acsmedchemlett.6b00369 BindingDB Entry DOI: 10.7270/Q24F1SQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205806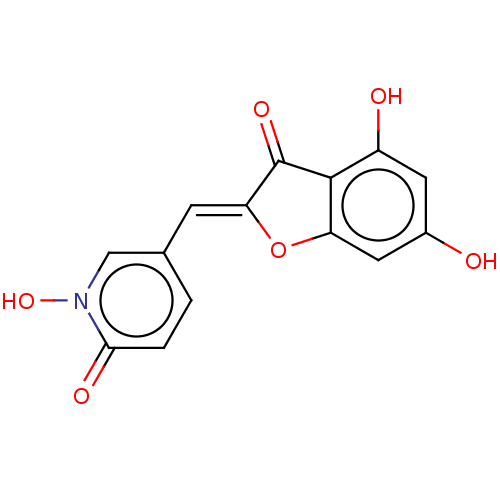 (CHEMBL3969839) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Grenoble-Alpes/CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells assessed as diphenolase activity using L-DOPA as substrate by ... | ACS Med Chem Lett 8: 55-60 (2017) Article DOI: 10.1021/acsmedchemlett.6b00369 BindingDB Entry DOI: 10.7270/Q24F1SQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205814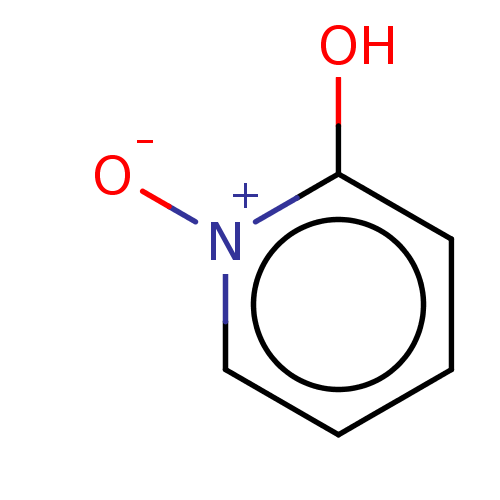 (CHEMBL3898657) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Grenoble-Alpes/CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells assessed as diphenolase activity using L-DOPA as substrate by ... | ACS Med Chem Lett 8: 55-60 (2017) Article DOI: 10.1021/acsmedchemlett.6b00369 BindingDB Entry DOI: 10.7270/Q24F1SQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Grenoble-Alpes/CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells assessed as diphenolase activity using L-DOPA as substrate by ... | ACS Med Chem Lett 8: 55-60 (2017) Article DOI: 10.1021/acsmedchemlett.6b00369 BindingDB Entry DOI: 10.7270/Q24F1SQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369335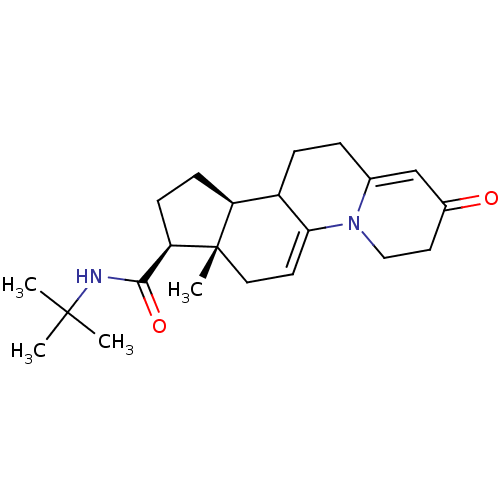 (CHEMBL1201841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate compared with finasteride in experiment 1 | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50057290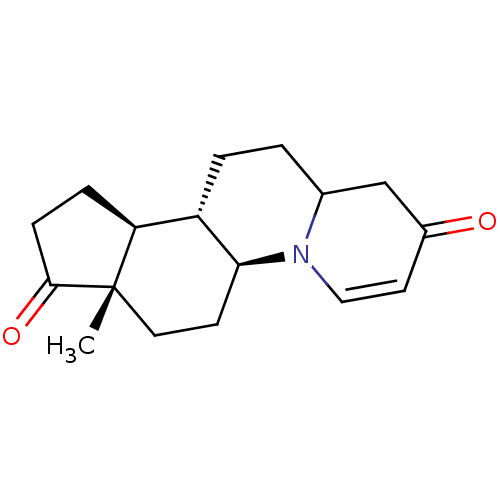 ((5aS,7aS,10aS,10bS)-7a-Methyl-1,5a,6,7,7a,9,10,10a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasteride | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50057297 ((5aS,7aS,8S,10aS,10bS)-8-Hydroxy-7a-methyl-3,4,6,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasteride | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50057289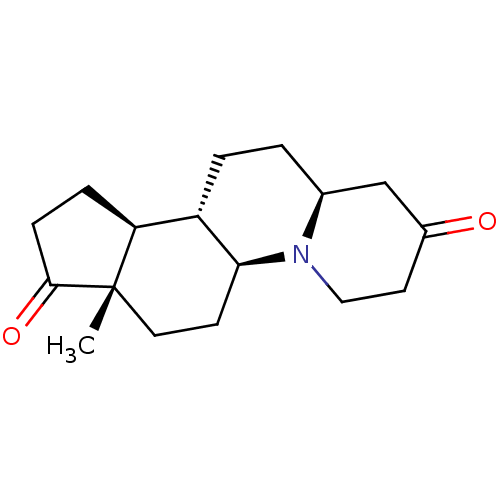 ((5aS,7aS,10aS,10bS,12aS)-7a-Methyl-tetradecahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasteride | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369338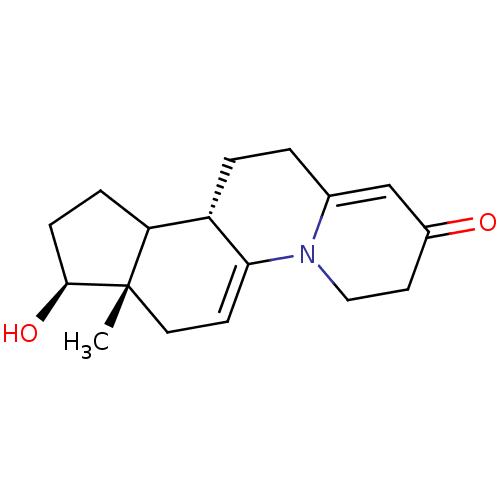 (CHEMBL1237294) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasteride | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissue | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50057292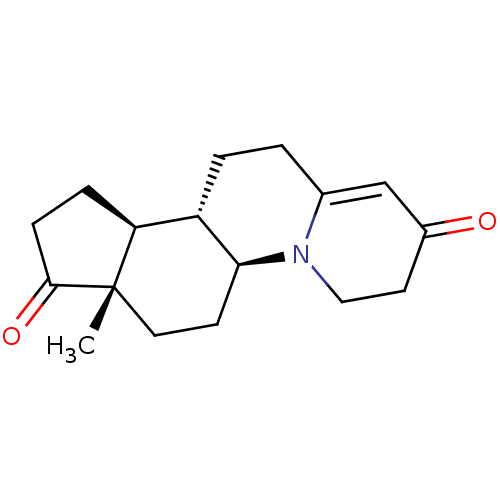 ((5aS,7aS,10aS,10bS)-7a-Methyl-3,4,5a,6,7,7a,9,10,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasteride | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369336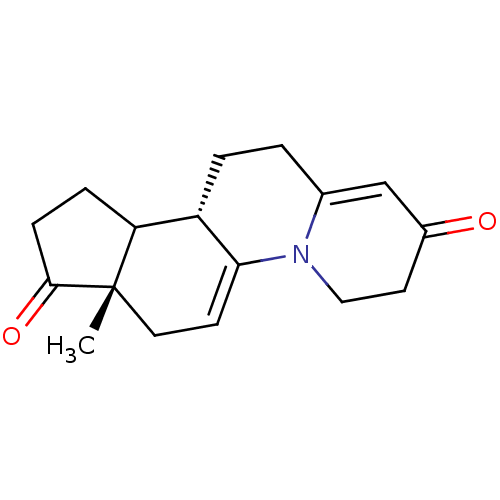 (CHEMBL1237306) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasteride | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369335 (CHEMBL1201841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate compared with finasteride in experiment 3 | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50057295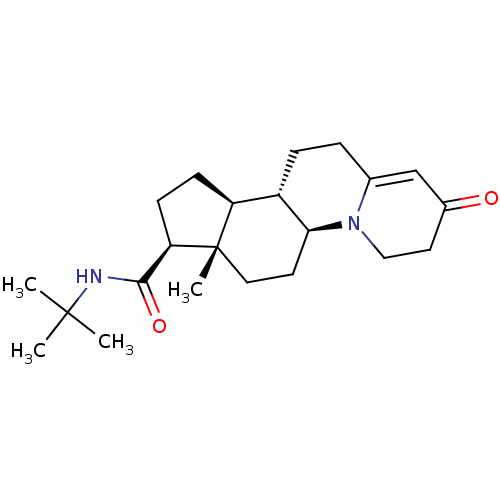 ((5aS,7aS,8S,10aS,10bS)-7a-Methyl-2-oxo-2,3,4,5a,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasteride | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369335 (CHEMBL1201841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate compared with finasteride in experiment 2 | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369334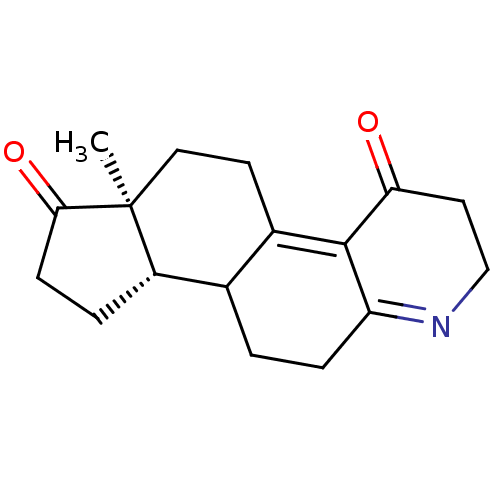 (CHEMBL1237307) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasteride | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369337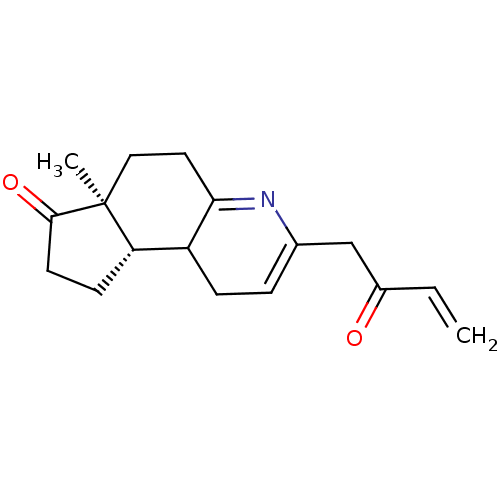 (CHEMBL1794821) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasteride | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369335 (CHEMBL1201841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Tested against 5 alpha R-2 on human prostate homogenate from surgically derived benign hyperplastic tissue in experiment 2 | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369335 (CHEMBL1201841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Tested against 5 alpha R-2 on human prostate homogenate from surgically derived benign hyperplastic tissue in experiment 1 | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369335 (CHEMBL1201841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissue in experiment 1 | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human 5-alpha Reductase-1 expressed in DU-145 cells | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369335 (CHEMBL1201841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description TInhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissue in experiment 2 | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50369335 (CHEMBL1201841) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human 5-alpha Reductase-1 expressed in DU-145 cells | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369335 (CHEMBL1201841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Tested against 5 alpha R-2 on human prostate homogenate from surgically derived benign hyperplastic tissue in experiment 3 | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50369336 (CHEMBL1237306) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human 5-alpha Reductase-1 expressed in DU-145 cells | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50057292 ((5aS,7aS,10aS,10bS)-7a-Methyl-3,4,5a,6,7,7a,9,10,1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 299 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human 5-alpha Reductase-1 expressed in DU-145 cells | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50057297 ((5aS,7aS,8S,10aS,10bS)-8-Hydroxy-7a-methyl-3,4,6,7...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 409 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human 5-alpha Reductase-1 expressed in DU-145 cells | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50057295 ((5aS,7aS,8S,10aS,10bS)-7a-Methyl-2-oxo-2,3,4,5a,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissue | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50057295 ((5aS,7aS,8S,10aS,10bS)-7a-Methyl-2-oxo-2,3,4,5a,6,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human 5-alpha Reductase-1 expressed in DU-145 cells | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177653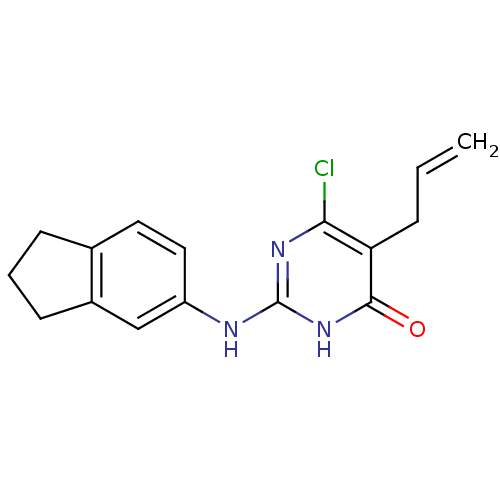 (5-allyl-6-chloro-2-(2,3-dihydro-1H-inden-5-ylamino...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177648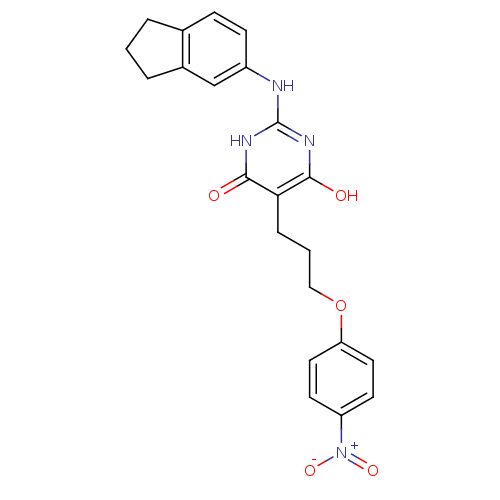 (2-(2,3-dihydro-1H-inden-5-ylamino)-6-hydroxy-5-(3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369338 (CHEMBL1237294) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissue | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177654 (4-(3-(2-(2,3-dihydro-1H-inden-5-ylamino)-4-hydroxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177644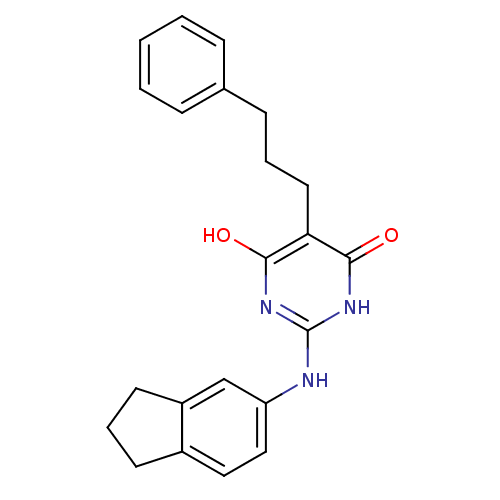 (2-(2,3-dihydro-1H-inden-5-ylamino)-6-hydroxy-5-(3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177645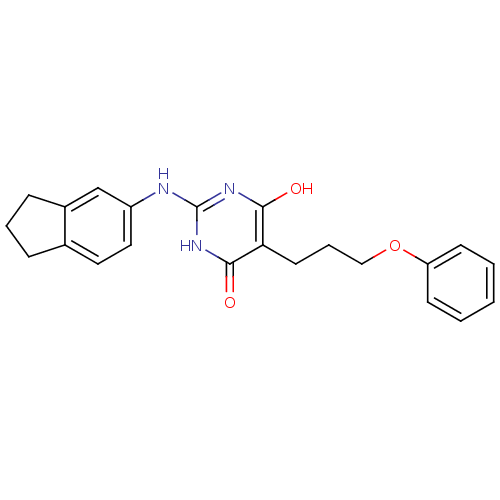 (2-(2,3-dihydro-1H-inden-5-ylamino)-6-hydroxy-5-(3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177651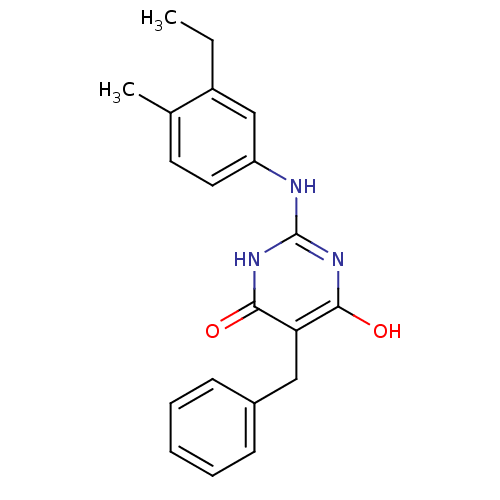 (5-benzyl-2-(3-ethyl-4-methylphenylamino)-6-hydroxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50057297 ((5aS,7aS,8S,10aS,10bS)-8-Hydroxy-7a-methyl-3,4,6,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissue | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50057292 ((5aS,7aS,10aS,10bS)-7a-Methyl-3,4,5a,6,7,7a,9,10,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissue | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369336 (CHEMBL1237306) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissue | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369336 (CHEMBL1237306) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissue | J Med Chem 40: 1112-29 (1997) Article DOI: 10.1021/jm960807v BindingDB Entry DOI: 10.7270/Q2D21Z8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177657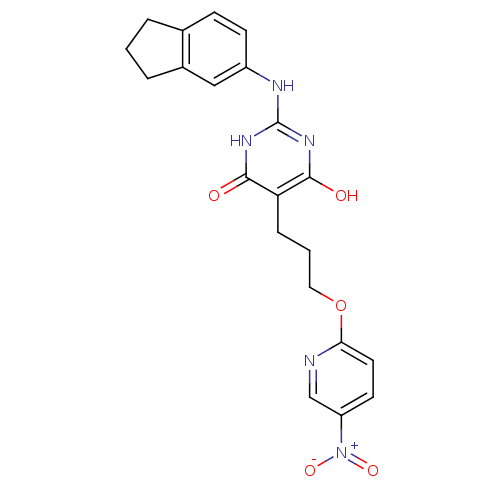 (2-(2,3-dihydro-1H-inden-5-ylamino)-6-hydroxy-5-(3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177647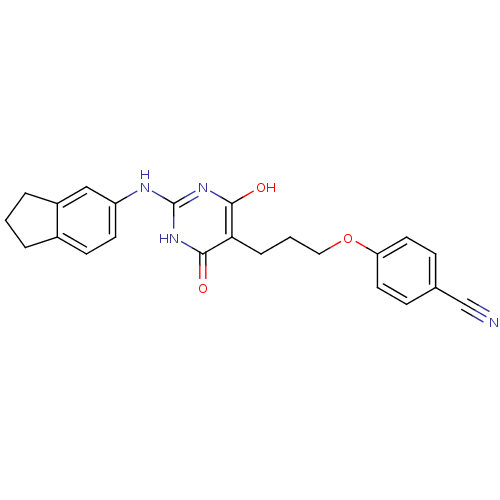 (4-(3-(2-(2,3-dihydro-1H-inden-5-ylamino)-4-hydroxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50378783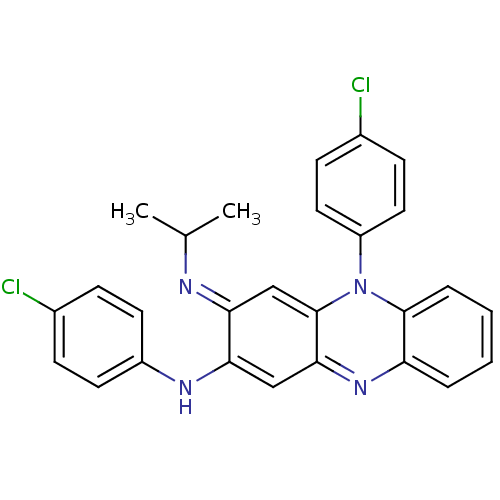 (CLOFAZIMINE) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata (UNLP) Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzaine in absence of 0.01% Triton | Eur J Med Chem 93: 338-48 (2015) Article DOI: 10.1016/j.ejmech.2015.01.065 BindingDB Entry DOI: 10.7270/Q2QR4ZV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50350995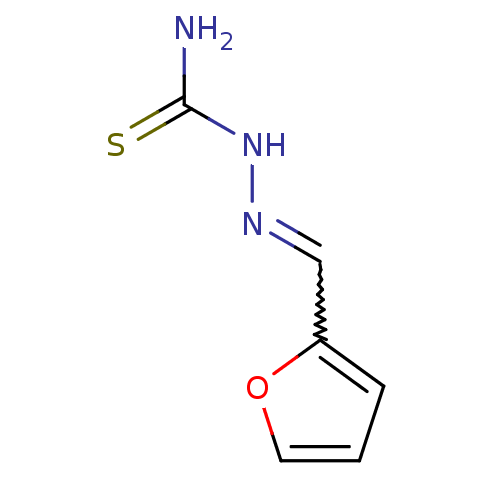 (CHEMBL1818876) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Grenoble/CNRS Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 mins by spectroscopic method | Eur J Med Chem 46: 4330-5 (2011) Article DOI: 10.1016/j.ejmech.2011.07.003 BindingDB Entry DOI: 10.7270/Q2SF2WKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50351005 (CHEMBL1818886) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Grenoble/CNRS Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 mins by spectroscopic method | Eur J Med Chem 46: 4330-5 (2011) Article DOI: 10.1016/j.ejmech.2011.07.003 BindingDB Entry DOI: 10.7270/Q2SF2WKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177665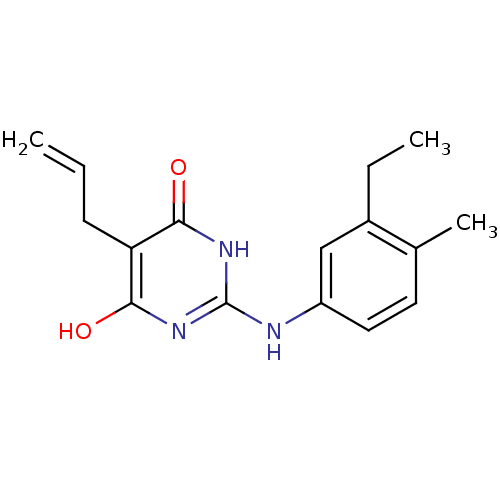 (5-allyl-2-(3-ethyl-4-methylphenylamino)-6-hydroxyp...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50350996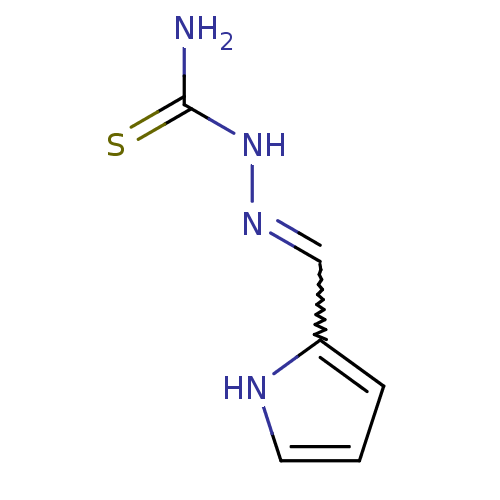 (CHEMBL1818877) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Grenoble/CNRS Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 mins by spectroscopic method | Eur J Med Chem 46: 4330-5 (2011) Article DOI: 10.1016/j.ejmech.2011.07.003 BindingDB Entry DOI: 10.7270/Q2SF2WKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50350993 (CHEMBL1818874) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Grenoble/CNRS Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 mins by spectroscopic method | Eur J Med Chem 46: 4330-5 (2011) Article DOI: 10.1016/j.ejmech.2011.07.003 BindingDB Entry DOI: 10.7270/Q2SF2WKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Staphylococcus aureus) | BDBM50177656 (5-(3-(4-aminophenoxy)propyl)-2-(2,3-dihydro-1H-ind...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Targanta Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of DNA polymerase3C from Staphylococcus aureus | Bioorg Med Chem Lett 16: 891-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.009 BindingDB Entry DOI: 10.7270/Q2125S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |