 Found 731 hits with Last Name = 'benowitz' and Initial = 'a'
Found 731 hits with Last Name = 'benowitz' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptide deformylase
(Escherichia coli) | BDBM50535516
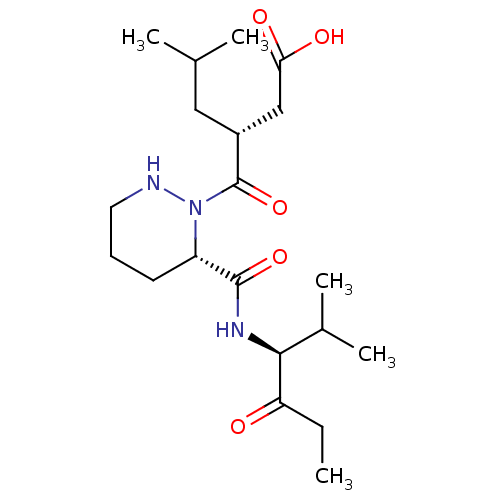
(CHEMBL4542964)Show SMILES CCC(=O)[C@@H](NC(=O)[C@@H]1CCCNN1C(=O)[C@H](CC(C)C)CC(O)=O)C(C)C |r| Show InChI InChI=1S/C20H35N3O5/c1-6-16(24)18(13(4)5)22-19(27)15-8-7-9-21-23(15)20(28)14(10-12(2)3)11-17(25)26/h12-15,18,21H,6-11H2,1-5H3,(H,22,27)(H,25,26)/t14-,15+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli PDF |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535514

(CHEMBL4458398)Show SMILES Cc1nc(NC(=O)[C@@H]2CCCNN2C(=O)[C@H](CC2CCCC2)CN(O)C=O)sc1C |r| Show InChI InChI=1S/C20H31N5O4S/c1-13-14(2)30-20(22-13)23-18(27)17-8-5-9-21-25(17)19(28)16(11-24(29)12-26)10-15-6-3-4-7-15/h12,15-17,21,29H,3-11H2,1-2H3,(H,22,23,27)/t16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535513

(CHEMBL4572153)Show SMILES CN(C)c1cc(NC(=O)[C@@H]2CCCNN2C(=O)[C@H](CC2CCCC2)CN(O)C=O)ncn1 |r| Show InChI InChI=1S/C21H33N7O4/c1-26(2)19-11-18(22-13-23-19)25-20(30)17-8-5-9-24-28(17)21(31)16(12-27(32)14-29)10-15-6-3-4-7-15/h11,13-17,24,32H,3-10,12H2,1-2H3,(H,22,23,25,30)/t16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by fluorescence detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535505

(CHEMBL4462876)Show SMILES ON(C[C@@H](CC1CCCC1)C(=O)N1NCCC[C@H]1C(=O)Nc1cnccn1)C=O |r| Show InChI InChI=1S/C19H28N6O4/c26-13-24(29)12-15(10-14-4-1-2-5-14)19(28)25-16(6-3-7-22-25)18(27)23-17-11-20-8-9-21-17/h8-9,11,13-16,22,29H,1-7,10,12H2,(H,21,23,27)/t15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535509

(CHEMBL4457234)Show SMILES CCc1nnc(NC(=O)[C@@H]2CCCNN2C(=O)[C@H](CC2CCCC2)CN(O)C=O)s1 |r| Show InChI InChI=1S/C19H30N6O4S/c1-2-16-22-23-19(30-16)21-17(27)15-8-5-9-20-25(15)18(28)14(11-24(29)12-26)10-13-6-3-4-7-13/h12-15,20,29H,2-11H2,1H3,(H,21,23,27)/t14-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535500

(CHEMBL4568739)Show SMILES ON(C[C@@H](CC1CCCC1)C(=O)N1NCCC[C@H]1C(=O)Nc1ccc(F)cn1)C=O |r| Show InChI InChI=1S/C20H28FN5O4/c21-16-7-8-18(22-11-16)24-19(28)17-6-3-9-23-26(17)20(29)15(12-25(30)13-27)10-14-4-1-2-5-14/h7-8,11,13-15,17,23,30H,1-6,9-10,12H2,(H,22,24,28)/t15-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535497

(CHEMBL4466084)Show SMILES ON(C[C@@H](CC1CCCC1)C(=O)N1NCCC[C@H]1C(=O)Nc1ccccn1)C=O |r| Show InChI InChI=1S/C20H29N5O4/c26-14-24(29)13-16(12-15-6-1-2-7-15)20(28)25-17(8-5-11-22-25)19(27)23-18-9-3-4-10-21-18/h3-4,9-10,14-17,22,29H,1-2,5-8,11-13H2,(H,21,23,27)/t16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535515

(CHEMBL4559778)Show SMILES Cc1ccnc(NC(=O)[C@@H]2CCCNN2C(=O)[C@H](CC2CCCC2)CN(O)C=O)c1 |r| Show InChI InChI=1S/C21H31N5O4/c1-15-8-10-22-19(11-15)24-20(28)18-7-4-9-23-26(18)21(29)17(13-25(30)14-27)12-16-5-2-3-6-16/h8,10-11,14,16-18,23,30H,2-7,9,12-13H2,1H3,(H,22,24,28)/t17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by fluorescence detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535518

(CHEMBL4448951)Show SMILES ON(C[C@@H](CC1CCCC1)C(=O)N1NCCC[C@H]1C(=O)Nc1cccnn1)C=O |r| Show InChI InChI=1S/C19H28N6O4/c26-13-24(29)12-15(11-14-5-1-2-6-14)19(28)25-16(7-3-10-21-25)18(27)22-17-8-4-9-20-23-17/h4,8-9,13-16,21,29H,1-3,5-7,10-12H2,(H,22,23,27)/t15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535512
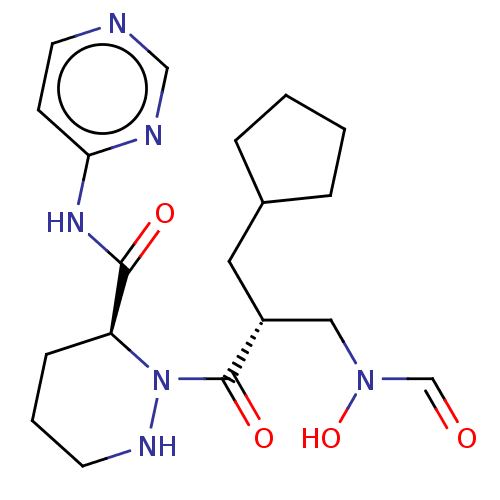
(CHEMBL4434997)Show SMILES ON(C[C@@H](CC1CCCC1)C(=O)N1NCCC[C@H]1C(=O)Nc1ccncn1)C=O |r| Show InChI InChI=1S/C19H28N6O4/c26-13-24(29)11-15(10-14-4-1-2-5-14)19(28)25-16(6-3-8-22-25)18(27)23-17-7-9-20-12-21-17/h7,9,12-16,22,29H,1-6,8,10-11H2,(H,20,21,23,27)/t15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535508
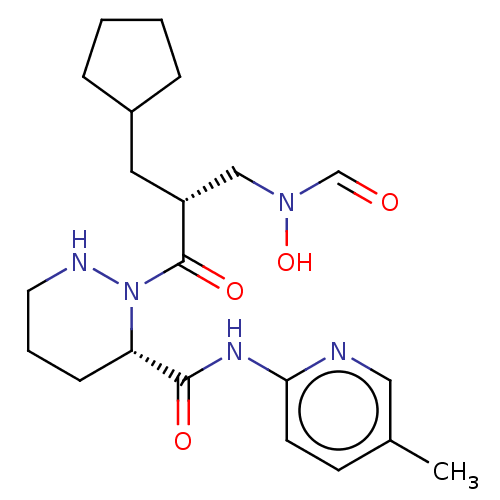
(CHEMBL4518477)Show SMILES Cc1ccc(NC(=O)[C@@H]2CCCNN2C(=O)[C@H](CC2CCCC2)CN(O)C=O)nc1 |r| Show InChI InChI=1S/C21H31N5O4/c1-15-8-9-19(22-12-15)24-20(28)18-7-4-10-23-26(18)21(29)17(13-25(30)14-27)11-16-5-2-3-6-16/h8-9,12,14,16-18,23,30H,2-7,10-11,13H2,1H3,(H,22,24,28)/t17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by fluorescence detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535504
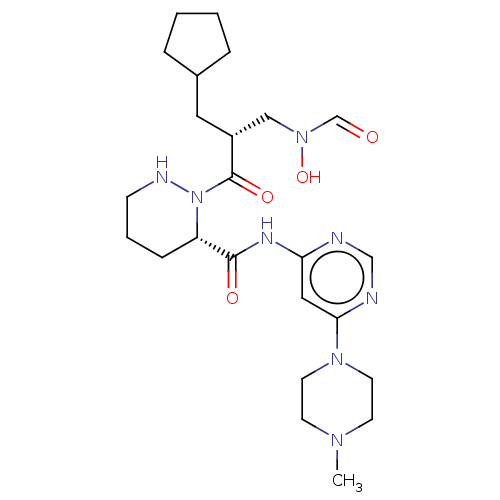
(CHEMBL4565584)Show SMILES CN1CCN(CC1)c1cc(NC(=O)[C@@H]2CCCNN2C(=O)[C@H](CC2CCCC2)CN(O)C=O)ncn1 |r| Show InChI InChI=1S/C24H38N8O4/c1-29-9-11-30(12-10-29)22-14-21(25-16-26-22)28-23(34)20-7-4-8-27-32(20)24(35)19(15-31(36)17-33)13-18-5-2-3-6-18/h14,16-20,27,36H,2-13,15H2,1H3,(H,25,26,28,34)/t19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by fluorescence detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50545382

(CHEMBL4646450)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCN(CCCCNC(=O)CNc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)CC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C43H49N11O7/c1-25-30-23-47-43(50-38(30)53(27-8-3-4-9-27)41(60)36(25)26(2)55)48-33-14-12-28(22-46-33)52-20-18-51(19-21-52)17-6-5-16-44-35(57)24-45-31-11-7-10-29-37(31)42(61)54(40(29)59)32-13-15-34(56)49-39(32)58/h7,10-12,14,22-23,27,32,45H,3-6,8-9,13,15-21,24H2,1-2H3,(H,44,57)(H,49,56,58)(H,46,47,48,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535511
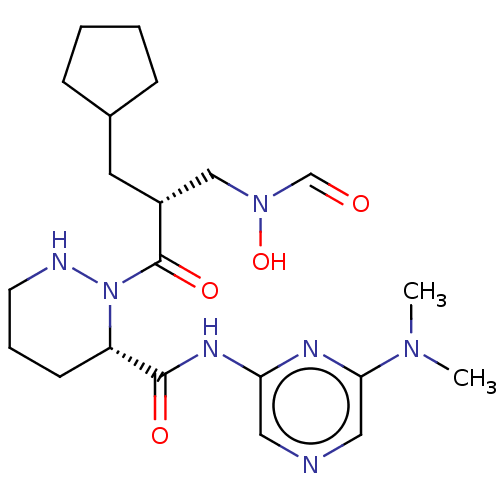
(CHEMBL4447270)Show SMILES CN(C)c1cncc(NC(=O)[C@@H]2CCCNN2C(=O)[C@H](CC2CCCC2)CN(O)C=O)n1 |r| Show InChI InChI=1S/C21H33N7O4/c1-26(2)19-12-22-11-18(24-19)25-20(30)17-8-5-9-23-28(17)21(31)16(13-27(32)14-29)10-15-6-3-4-7-15/h11-12,14-17,23,32H,3-10,13H2,1-2H3,(H,24,25,30)/t16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by fluorescence detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50545379
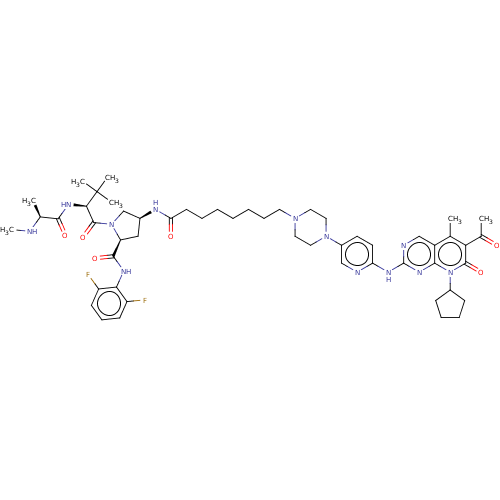
(CHEMBL4645010)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)Nc1c(F)cccc1F)NC(=O)CCCCCCCN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C |r| Show InChI InChI=1S/C53H72F2N12O6/c1-32-38-30-58-52(63-47(38)67(36-16-12-13-17-36)50(72)44(32)34(3)68)60-42-22-21-37(29-57-42)65-26-24-64(25-27-65)23-14-10-8-9-11-20-43(69)59-35-28-41(49(71)61-45-39(54)18-15-19-40(45)55)66(31-35)51(73)46(53(4,5)6)62-48(70)33(2)56-7/h15,18-19,21-22,29-30,33,35-36,41,46,56H,8-14,16-17,20,23-28,31H2,1-7H3,(H,59,69)(H,61,71)(H,62,70)(H,57,58,60,63)/t33-,35-,41-,46+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535501

(CHEMBL4435970)Show SMILES ON(C[C@@H](CC1CCCC1)C(=O)N1NCCC[C@H]1C(=O)Nc1ccccc1)C=O |r| Show InChI InChI=1S/C21H30N4O4/c26-15-24(29)14-17(13-16-7-4-5-8-16)21(28)25-19(11-6-12-22-25)20(27)23-18-9-2-1-3-10-18/h1-3,9-10,15-17,19,22,29H,4-8,11-14H2,(H,23,27)/t17-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535499

(CHEMBL4437963)Show SMILES ON(C[C@@H](CC1CCCC1)C(=O)N1NCCC[C@H]1C(=O)N1CCCCC1)C=O |r| Show InChI InChI=1S/C20H34N4O4/c25-15-23(28)14-17(13-16-7-2-3-8-16)19(26)24-18(9-6-10-21-24)20(27)22-11-4-1-5-12-22/h15-18,21,28H,1-14H2/t17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50545380
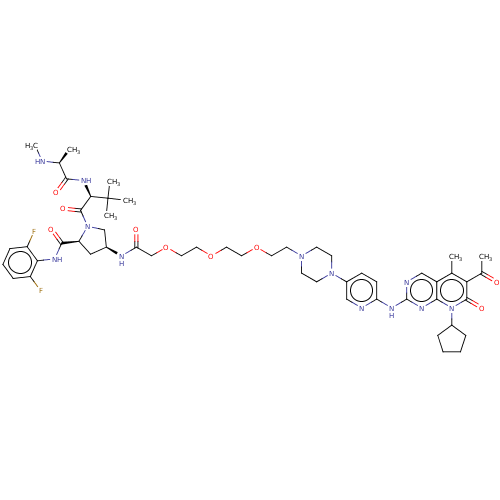
(CHEMBL4645458)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)Nc1c(F)cccc1F)NC(=O)COCCOCCOCCN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C |r| Show InChI InChI=1S/C53H72F2N12O9/c1-32-38-29-58-52(63-47(38)67(36-11-8-9-12-36)50(72)44(32)34(3)68)60-42-16-15-37(28-57-42)65-19-17-64(18-20-65)21-22-74-23-24-75-25-26-76-31-43(69)59-35-27-41(49(71)61-45-39(54)13-10-14-40(45)55)66(30-35)51(73)46(53(4,5)6)62-48(70)33(2)56-7/h10,13-16,28-29,33,35-36,41,46,56H,8-9,11-12,17-27,30-31H2,1-7H3,(H,59,69)(H,61,71)(H,62,70)(H,57,58,60,63)/t33-,35-,41-,46+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50545383
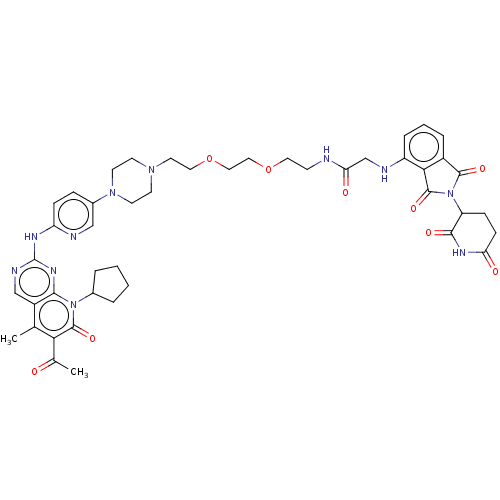
(CHEMBL4636355)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCN(CCOCCOCCNC(=O)CNc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)CC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C45H53N11O9/c1-27-32-25-49-45(52-40(32)55(29-6-3-4-7-29)43(62)38(27)28(2)57)50-35-12-10-30(24-48-35)54-17-15-53(16-18-54)19-21-65-23-22-64-20-14-46-37(59)26-47-33-9-5-8-31-39(33)44(63)56(42(31)61)34-11-13-36(58)51-41(34)60/h5,8-10,12,24-25,29,34,47H,3-4,6-7,11,13-23,26H2,1-2H3,(H,46,59)(H,51,58,60)(H,48,49,50,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50545382

(CHEMBL4646450)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCN(CCCCNC(=O)CNc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)CC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C43H49N11O7/c1-25-30-23-47-43(50-38(30)53(27-8-3-4-9-27)41(60)36(25)26(2)55)48-33-14-12-28(22-46-33)52-20-18-51(19-21-52)17-6-5-16-44-35(57)24-45-31-11-7-10-29-37(31)42(61)54(40(29)59)32-13-15-34(56)49-39(32)58/h7,10-12,14,22-23,27,32,45H,3-6,8-9,13,15-21,24H2,1-2H3,(H,44,57)(H,49,56,58)(H,46,47,48,50) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6309
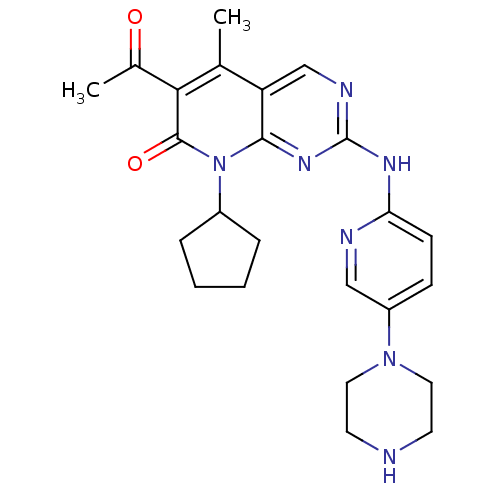
(6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535503
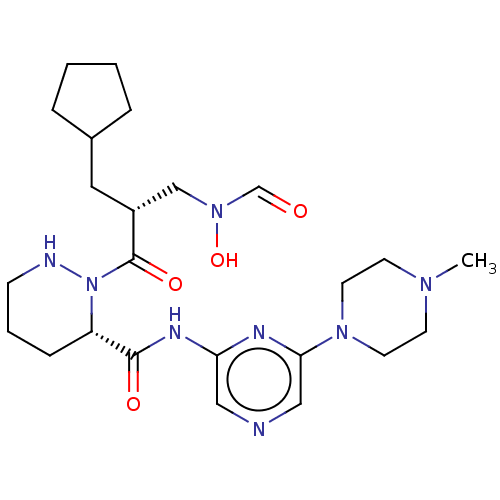
(CHEMBL4454464)Show SMILES CN1CCN(CC1)c1cncc(NC(=O)[C@@H]2CCCNN2C(=O)[C@H](CC2CCCC2)CN(O)C=O)n1 |r| Show InChI InChI=1S/C24H38N8O4/c1-29-9-11-30(12-10-29)22-15-25-14-21(27-22)28-23(34)20-7-4-8-26-32(20)24(35)19(16-31(36)17-33)13-18-5-2-3-6-18/h14-15,17-20,26,36H,2-13,16H2,1H3,(H,27,28,34)/t19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by fluorescence detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535506
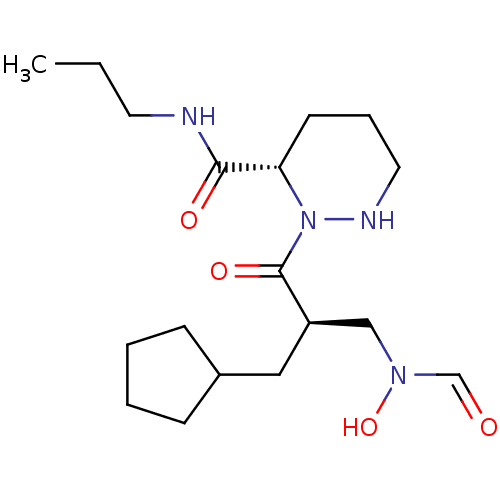
(CHEMBL4440847)Show SMILES CCCNC(=O)[C@@H]1CCCNN1C(=O)[C@H](CC1CCCC1)CN(O)C=O |r| Show InChI InChI=1S/C18H32N4O4/c1-2-9-19-17(24)16-8-5-10-20-22(16)18(25)15(12-21(26)13-23)11-14-6-3-4-7-14/h13-16,20,26H,2-12H2,1H3,(H,19,24)/t15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535502
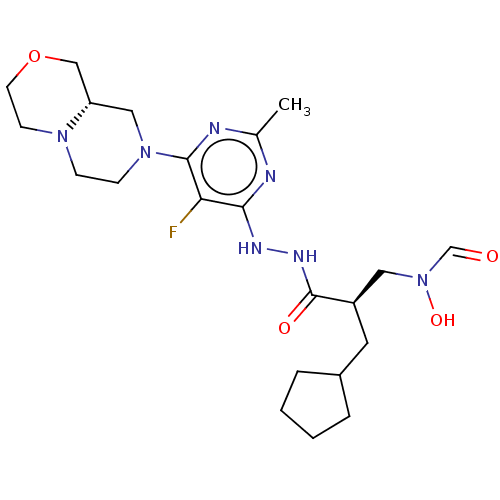
(GSK-1322322B | GSK1322322 | GSK1322322B | Lanopepd...)Show SMILES [H][C@@](CC1CCCC1)(CN(O)C=O)C(=O)NNc1nc(C)nc(N2CCN3CCOC[C@]3([H])C2)c1F |r| Show InChI InChI=1S/C22H34FN7O4/c1-15-24-20(19(23)21(25-15)29-7-6-28-8-9-34-13-18(28)12-29)26-27-22(32)17(11-30(33)14-31)10-16-4-2-3-5-16/h14,16-18,33H,2-13H2,1H3,(H,27,32)(H,24,25,26)/t17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50545379

(CHEMBL4645010)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)Nc1c(F)cccc1F)NC(=O)CCCCCCCN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C |r| Show InChI InChI=1S/C53H72F2N12O6/c1-32-38-30-58-52(63-47(38)67(36-16-12-13-17-36)50(72)44(32)34(3)68)60-42-22-21-37(29-57-42)65-26-24-64(25-27-65)23-14-10-8-9-11-20-43(69)59-35-28-41(49(71)61-45-39(54)18-15-19-40(45)55)66(31-35)51(73)46(53(4,5)6)62-48(70)33(2)56-7/h15,18-19,21-22,29-30,33,35-36,41,46,56H,8-14,16-17,20,23-28,31H2,1-7H3,(H,59,69)(H,61,71)(H,62,70)(H,57,58,60,63)/t33-,35-,41-,46+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
DNA ligase
(Staphylococcus aureus) | BDBM50444582

(CHEMBL3099715)Show InChI InChI=1S/C13H11F3N6O/c14-13(15,16)9-5-10(18-3-4-23)20-12(19-9)11-7-1-2-17-6-8(7)21-22-11/h1-2,5-6,23H,3-4H2,(H,21,22)(H,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal 6xHis-tagged full length recombinant Staphylococcus aureus DNA ligase (1 to 312) expressed in Escherichia coli BL21 (DE3) us... |
ACS Med Chem Lett 4: 1208-12 (2013)
Article DOI: 10.1021/ml4003277
BindingDB Entry DOI: 10.7270/Q2J967V2 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535498
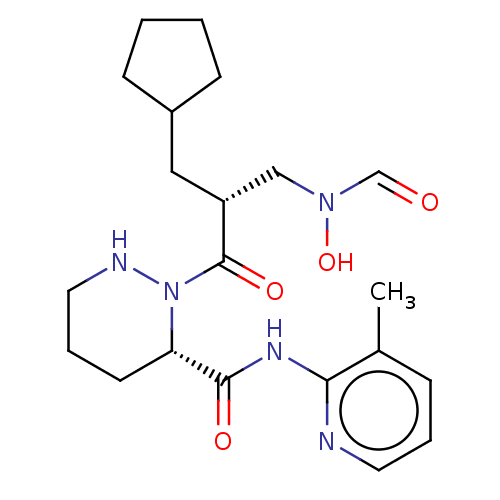
(CHEMBL4435803)Show SMILES Cc1cccnc1NC(=O)[C@@H]1CCCNN1C(=O)[C@H](CC1CCCC1)CN(O)C=O |r| Show InChI InChI=1S/C21H31N5O4/c1-15-6-4-10-22-19(15)24-20(28)18-9-5-11-23-26(18)21(29)17(13-25(30)14-27)12-16-7-2-3-8-16/h4,6,10,14,16-18,23,30H,2-3,5,7-9,11-13H2,1H3,(H,22,24,28)/t17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by fluorescence detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50545383

(CHEMBL4636355)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCN(CCOCCOCCNC(=O)CNc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)CC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C45H53N11O9/c1-27-32-25-49-45(52-40(32)55(29-6-3-4-7-29)43(62)38(27)28(2)57)50-35-12-10-30(24-48-35)54-17-15-53(16-18-54)19-21-65-23-22-64-20-14-46-37(59)26-47-33-9-5-8-31-39(33)44(63)56(42(31)61)34-11-13-36(58)51-41(34)60/h5,8-10,12,24-25,29,34,47H,3-4,6-7,11,13-23,26H2,1-2H3,(H,46,59)(H,51,58,60)(H,48,49,50,52) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50545380

(CHEMBL4645458)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)Nc1c(F)cccc1F)NC(=O)COCCOCCOCCN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C |r| Show InChI InChI=1S/C53H72F2N12O9/c1-32-38-29-58-52(63-47(38)67(36-11-8-9-12-36)50(72)44(32)34(3)68)60-42-16-15-37(28-57-42)65-19-17-64(18-20-65)21-22-74-23-24-75-25-26-76-31-43(69)59-35-27-41(49(71)61-45-39(54)13-10-14-40(45)55)66(30-35)51(73)46(53(4,5)6)62-48(70)33(2)56-7/h10,13-16,28-29,33,35-36,41,46,56H,8-9,11-12,17-27,30-31H2,1-7H3,(H,59,69)(H,61,71)(H,62,70)(H,57,58,60,63)/t33-,35-,41-,46+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
G1/S-specific cyclin-D2
(Homo sapiens (Human)) | BDBM6309

(6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D2 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50545381
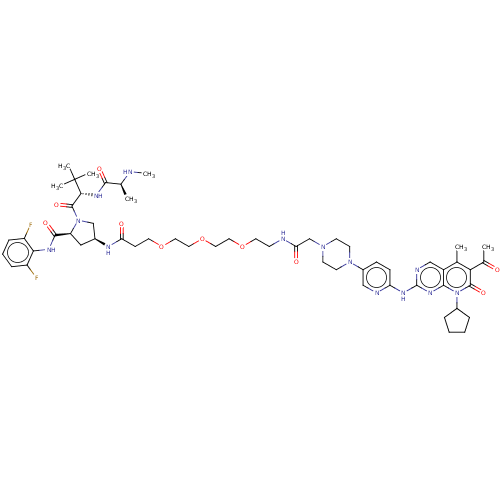
(CHEMBL4649032)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)Nc1c(F)cccc1F)NC(=O)CCOCCOCCOCCNC(=O)CN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C |r| Show InChI InChI=1S/C56H77F2N13O10/c1-34-40-31-62-55(67-50(40)71(38-11-8-9-12-38)53(77)47(34)36(3)72)64-44-16-15-39(30-61-44)69-21-19-68(20-22-69)33-46(74)60-18-24-80-26-28-81-27-25-79-23-17-45(73)63-37-29-43(52(76)65-48-41(57)13-10-14-42(48)58)70(32-37)54(78)49(56(4,5)6)66-51(75)35(2)59-7/h10,13-16,30-31,35,37-38,43,49,59H,8-9,11-12,17-29,32-33H2,1-7H3,(H,60,74)(H,63,73)(H,65,76)(H,66,75)(H,61,62,64,67)/t35-,37-,43-,49+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535510
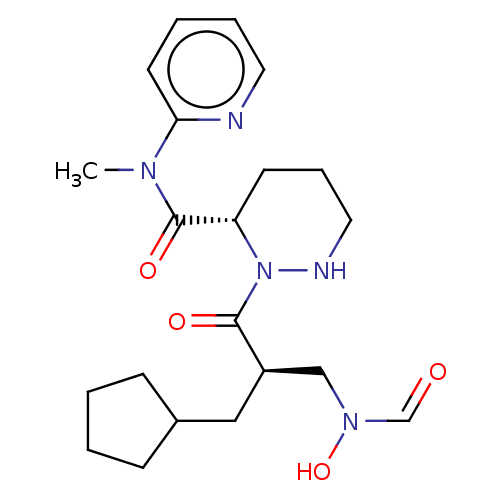
(CHEMBL4471518)Show SMILES CN(C(=O)[C@@H]1CCCNN1C(=O)[C@H](CC1CCCC1)CN(O)C=O)c1ccccn1 |r| Show InChI InChI=1S/C21H31N5O4/c1-24(19-10-4-5-11-22-19)21(29)18-9-6-12-23-26(18)20(28)17(14-25(30)15-27)13-16-7-2-3-8-16/h4-5,10-11,15-18,23,30H,2-3,6-9,12-14H2,1H3/t17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by absorbance detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535507
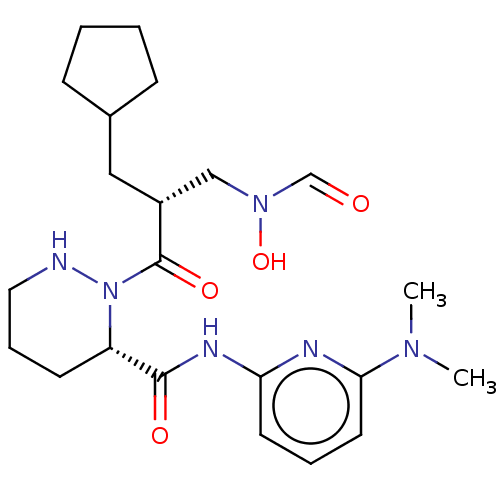
(CHEMBL4547624)Show SMILES CN(C)c1cccc(NC(=O)[C@@H]2CCCNN2C(=O)[C@H](CC2CCCC2)CN(O)C=O)n1 |r| Show InChI InChI=1S/C22H34N6O4/c1-26(2)20-11-5-10-19(24-20)25-21(30)18-9-6-12-23-28(18)22(31)17(14-27(32)15-29)13-16-7-3-4-8-16/h5,10-11,15-18,23,32H,3-4,6-9,12-14H2,1-2H3,(H,24,25,30)/t17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by fluorescence detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50545384
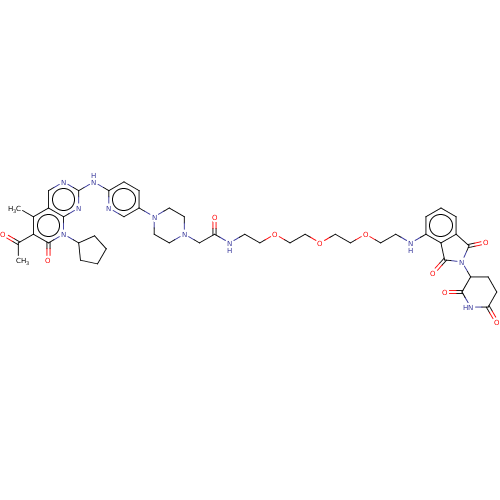
(CHEMBL4548417)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCN(CC(=O)NCCOCCOCCOCCNc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)CC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C47H57N11O10/c1-29-34-27-51-47(54-42(34)57(31-6-3-4-7-31)45(64)40(29)30(2)59)52-37-12-10-32(26-50-37)56-18-16-55(17-19-56)28-39(61)49-15-21-67-23-25-68-24-22-66-20-14-48-35-9-5-8-33-41(35)46(65)58(44(33)63)36-11-13-38(60)53-43(36)62/h5,8-10,12,26-27,31,36,48H,3-4,6-7,11,13-25,28H2,1-2H3,(H,49,61)(H,53,60,62)(H,50,51,52,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50545378
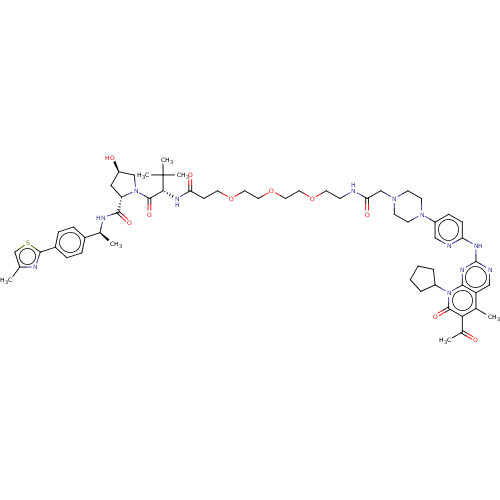
(CHEMBL4638298)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)CCOCCOCCOCCNC(=O)CN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C)c1ccc(cc1)-c1nc(C)cs1 |r| Show InChI InChI=1S/C58H78N12O10S/c1-36-35-81-54(62-36)41-14-12-40(13-15-41)38(3)63-53(75)46-30-44(72)33-69(46)56(77)51(58(5,6)7)65-48(73)18-24-78-26-28-80-29-27-79-25-19-59-49(74)34-67-20-22-68(23-21-67)43-16-17-47(60-31-43)64-57-61-32-45-37(2)50(39(4)71)55(76)70(52(45)66-57)42-10-8-9-11-42/h12-17,31-32,35,38,42,44,46,51,72H,8-11,18-30,33-34H2,1-7H3,(H,59,74)(H,63,75)(H,65,73)(H,60,61,64,66)/t38-,44+,46-,51+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50545376
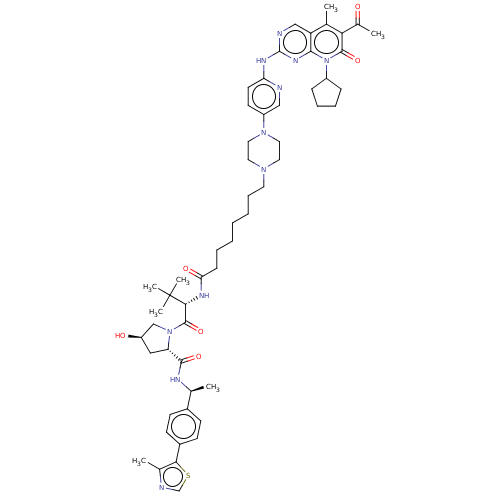
(CHEMBL4638166)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)CCCCCCCN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C)c1ccc(cc1)-c1scnc1C |r| Show InChI InChI=1S/C55H73N11O6S/c1-34-43-31-57-54(62-50(43)66(40-15-12-13-16-40)52(71)47(34)37(4)67)60-45-23-22-41(30-56-45)64-27-25-63(26-28-64)24-14-10-8-9-11-17-46(69)61-49(55(5,6)7)53(72)65-32-42(68)29-44(65)51(70)59-35(2)38-18-20-39(21-19-38)48-36(3)58-33-73-48/h18-23,30-31,33,35,40,42,44,49,68H,8-17,24-29,32H2,1-7H3,(H,59,70)(H,61,69)(H,56,57,60,62)/t35-,42+,44-,49+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6309

(6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50545377
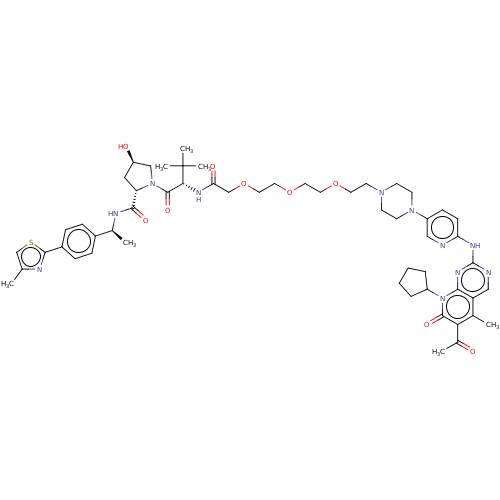
(CHEMBL4640158)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)COCCOCCOCCN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C)c1ccc(cc1)-c1nc(C)cs1 |r| Show InChI InChI=1S/C55H73N11O9S/c1-34-33-76-51(58-34)39-14-12-38(13-15-39)36(3)59-50(70)44-28-42(68)31-65(44)53(72)48(55(5,6)7)61-46(69)32-75-27-26-74-25-24-73-23-22-63-18-20-64(21-19-63)41-16-17-45(56-29-41)60-54-57-30-43-35(2)47(37(4)67)52(71)66(49(43)62-54)40-10-8-9-11-40/h12-17,29-30,33,36,40,42,44,48,68H,8-11,18-28,31-32H2,1-7H3,(H,59,70)(H,61,69)(H,56,57,60,62)/t36-,42+,44-,48+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50545381

(CHEMBL4649032)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)Nc1c(F)cccc1F)NC(=O)CCOCCOCCOCCNC(=O)CN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C |r| Show InChI InChI=1S/C56H77F2N13O10/c1-34-40-31-62-55(67-50(40)71(38-11-8-9-12-38)53(77)47(34)36(3)72)64-44-16-15-39(30-61-44)69-21-19-68(20-22-69)33-46(74)60-18-24-80-26-28-81-27-25-79-23-17-45(73)63-37-29-43(52(76)65-48-41(57)13-10-14-42(48)58)70(32-37)54(78)49(56(4,5)6)66-51(75)35(2)59-7/h10,13-16,30-31,35,37-38,43,49,59H,8-9,11-12,17-29,32-33H2,1-7H3,(H,60,74)(H,63,73)(H,65,76)(H,66,75)(H,61,62,64,67)/t35-,37-,43-,49+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50545384

(CHEMBL4548417)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCN(CC(=O)NCCOCCOCCOCCNc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)CC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C47H57N11O10/c1-29-34-27-51-47(54-42(34)57(31-6-3-4-7-31)45(64)40(29)30(2)59)52-37-12-10-32(26-50-37)56-18-16-55(17-19-56)28-39(61)49-15-21-67-23-25-68-24-22-66-20-14-48-35-9-5-8-33-41(35)46(65)58(44(33)63)36-11-13-38(60)53-43(36)62/h5,8-10,12,26-27,31,36,48H,3-4,6-7,11,13-25,28H2,1-2H3,(H,49,61)(H,53,60,62)(H,50,51,52,54) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM621246
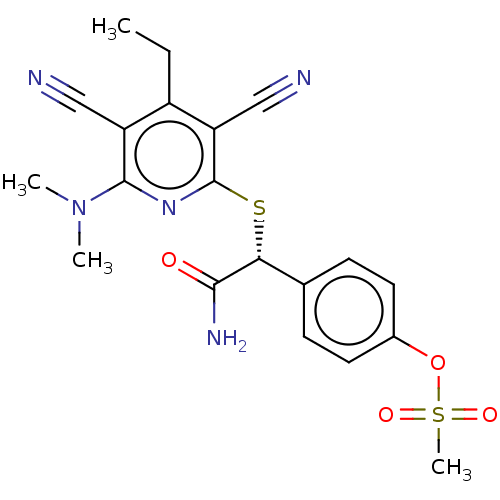
(US11771711, Example 2)Show SMILES CCc1c(C#N)c(S[C@@H](C(N)=O)c2ccc(OS(C)(=O)=O)cc2)nc(N(C)C)c1C#N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1 [601-1600]
(Homo sapiens (Human)) | BDBM491448

(US10975056, Example 422 | US11771711, Reference co...)Show SMILES CCc1c(C#N)c(S[C@@H](C(N)=O)c2ccc(OC)cc2)nc(N2CC[C@H](O)C2)c1C#N |r| Show InChI InChI=1S/C22H23N5O3S/c1-3-16-17(10-23)21(27-9-8-14(28)12-27)26-22(18(16)11-24)31-19(20(25)29)13-4-6-15(30-2)7-5-13/h4-7,14,19,28H,3,8-9,12H2,1-2H3,(H2,25,29)/t14-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
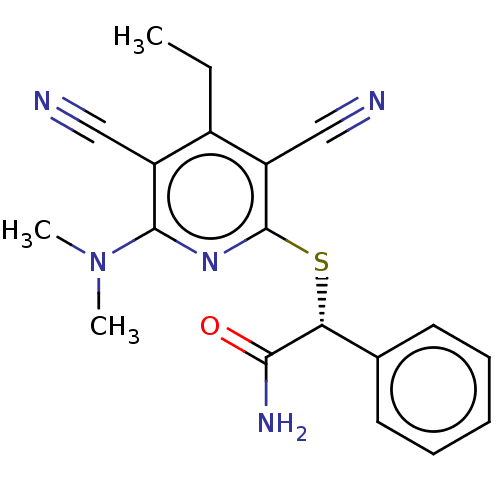
(Homo sapiens (Human)) | BDBM491120
((R)-2-((3,5-dicyano-6-(dimethylamino)-4-ethylpyrid...)Show SMILES CCc1c(C#N)c(S[C@@H](C(N)=O)c2ccccc2)nc(N(C)C)c1C#N |r| Show InChI InChI=1S/C19H19N5OS/c1-4-13-14(10-20)18(24(2)3)23-19(15(13)11-21)26-16(17(22)25)12-8-6-5-7-9-12/h5-9,16H,4H2,1-3H3,(H2,22,25)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM6309

(6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM621248

(4-(2-amino-1-((3,5-dicyano-6-(dimethylamino)-4-eth...)Show SMILES CCc1c(C#N)c(SC(C(N)=O)c2ccc(OS(C)(=O)=O)cc2)nc(N(C)C)c1C#N | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50545378

(CHEMBL4638298)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)CCOCCOCCOCCNC(=O)CN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C)c1ccc(cc1)-c1nc(C)cs1 |r| Show InChI InChI=1S/C58H78N12O10S/c1-36-35-81-54(62-36)41-14-12-40(13-15-41)38(3)63-53(75)46-30-44(72)33-69(46)56(77)51(58(5,6)7)65-48(73)18-24-78-26-28-80-29-27-79-25-19-59-49(74)34-67-20-22-68(23-21-67)43-16-17-47(60-31-43)64-57-61-32-45-37(2)50(39(4)71)55(76)70(52(45)66-57)42-10-8-9-11-42/h12-17,31-32,35,38,42,44,46,51,72H,8-11,18-30,33-34H2,1-7H3,(H,59,74)(H,63,75)(H,65,73)(H,60,61,64,66)/t38-,44+,46-,51+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM491224

(US10975056, Example 168 | US11771711, Reference co...)Show SMILES CCc1c(C#N)c(S[C@@H](C(N)=O)c2ccccc2)nc(N2CC[C@H](O)C2)c1C#N |r| Show InChI InChI=1S/C21H21N5O2S/c1-2-15-16(10-22)20(26-9-8-14(27)12-26)25-21(17(15)11-23)29-18(19(24)28)13-6-4-3-5-7-13/h3-7,14,18,27H,2,8-9,12H2,1H3,(H2,24,28)/t14-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50535517
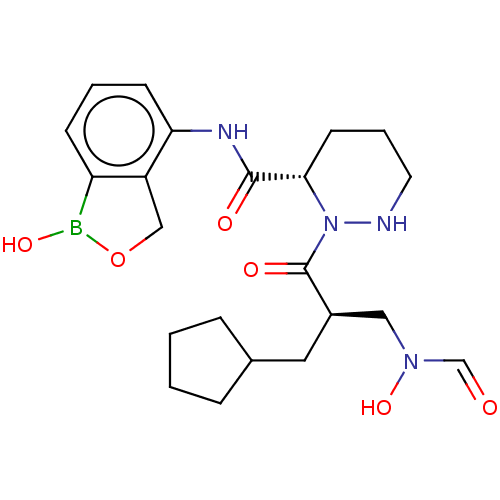
(CHEMBL4474270)Show SMILES OB1OCc2c1cccc2NC(=O)[C@@H]1CCCNN1C(=O)[C@H](CC1CCCC1)CN(O)C=O |r| Show InChI InChI=1S/C22H31BN4O6/c28-14-26(32)12-16(11-15-5-1-2-6-15)22(30)27-20(9-4-10-24-27)21(29)25-19-8-3-7-18-17(19)13-33-23(18)31/h3,7-8,14-16,20,24,31-32H,1-2,4-6,9-13H2,(H,25,29)/t16-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus PDF by fluorescence detection assay |
Bioorg Med Chem Lett 29: 2410-2414 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.028
BindingDB Entry DOI: 10.7270/Q2FF3WV7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50545377

(CHEMBL4640158)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)COCCOCCOCCN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C)c1ccc(cc1)-c1nc(C)cs1 |r| Show InChI InChI=1S/C55H73N11O9S/c1-34-33-76-51(58-34)39-14-12-38(13-15-39)36(3)59-50(70)44-28-42(68)31-65(44)53(72)48(55(5,6)7)61-46(69)32-75-27-26-74-25-24-73-23-22-63-18-20-64(21-19-63)41-16-17-45(56-29-41)60-54-57-30-43-35(2)47(37(4)67)52(71)66(49(43)62-54)40-10-8-9-11-40/h12-17,29-30,33,36,40,42,44,48,68H,8-11,18-28,31-32H2,1-7H3,(H,59,70)(H,61,69)(H,56,57,60,62)/t36-,42+,44-,48+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50545376

(CHEMBL4638166)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)CCCCCCCN1CCN(CC1)c1ccc(Nc2ncc3c(C)c(C(C)=O)c(=O)n(C4CCCC4)c3n2)nc1)C(C)(C)C)c1ccc(cc1)-c1scnc1C |r| Show InChI InChI=1S/C55H73N11O6S/c1-34-43-31-57-54(62-50(43)66(40-15-12-13-16-40)52(71)47(34)37(4)67)60-45-23-22-41(30-56-45)64-27-25-63(26-28-64)24-14-10-8-9-11-17-46(69)61-49(55(5,6)7)53(72)65-32-42(68)29-44(65)51(70)59-35(2)38-18-20-39(21-19-38)48-36(3)58-33-73-48/h18-23,30-31,33,35,40,42,44,49,68H,8-17,24-29,32H2,1-7H3,(H,59,70)(H,61,69)(H,56,57,60,62)/t35-,42+,44-,49+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127106
BindingDB Entry DOI: 10.7270/Q2JW8JF1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data