

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C alpha/beta/gamma/delta/epsilon/eta/theta type/Serine/threonine-protein kinase D1/D3 (Homo sapiens (Human)) | BDBM50368315 (PROSTRATIN) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute (NCI) Curated by ChEMBL | Assay Description Displacement of [3H]PDBu binding to Protein kinase C of CEM cells with 10% fetal calf serum | J Med Chem 35: 1978-86 (1992) BindingDB Entry DOI: 10.7270/Q22Z17SG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C alpha/beta/gamma/delta/epsilon/eta/theta type/Serine/threonine-protein kinase D1/D3 (Homo sapiens (Human)) | BDBM50368315 (PROSTRATIN) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute (NCI) Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from Protein kinase C of CEM cells | J Med Chem 35: 1978-86 (1992) BindingDB Entry DOI: 10.7270/Q22Z17SG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50494419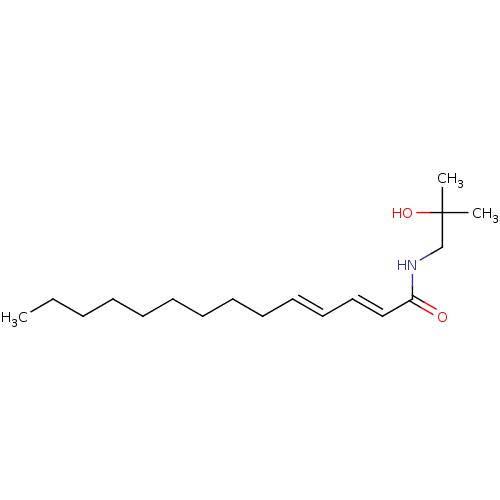 (Tetrahydrobungeanool) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after 1 h... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482161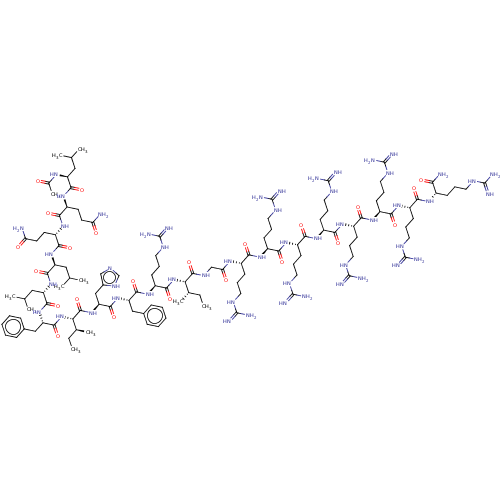 (CHEMBL1082256) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482160 (CHEMBL1082257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482160 (CHEMBL1082257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-end processing activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482161 (CHEMBL1082256) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-end processing activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50494416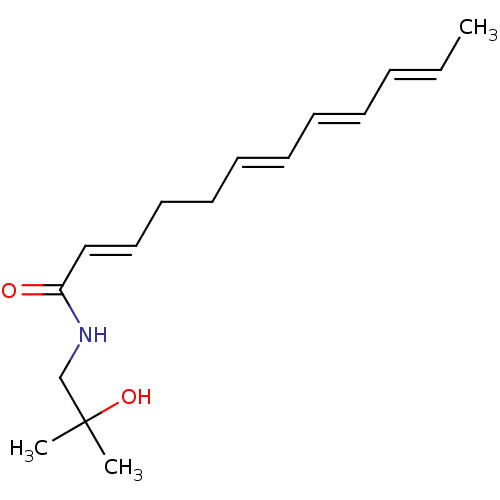 (CHEMBL2335715 | Hydroxy-Beta-Sanshool) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-Win55212-2 from human CB1 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after ... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50478518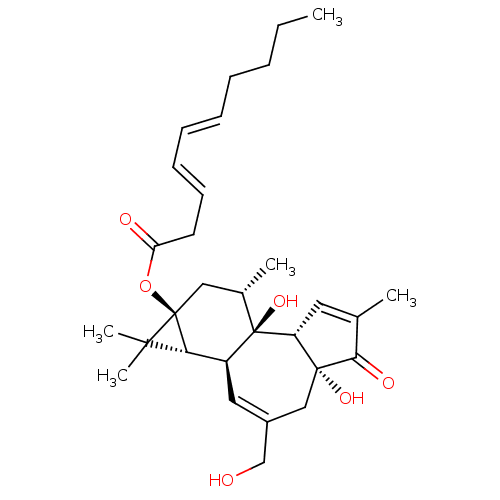 (CHEMBL514017) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from rat brain membrane PKC | J Nat Prod 58: 769-72 (1995) Article DOI: 10.1021/np50119a020 BindingDB Entry DOI: 10.7270/Q2NZ8BF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor Jun (Homo sapiens (Human)) | BDBM50295171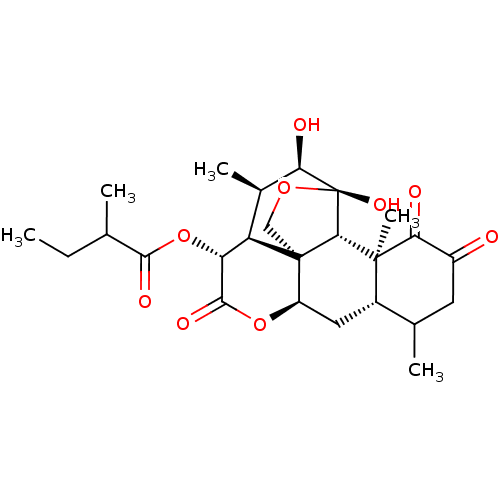 (AILANTHINONE | CHEMBL487194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of TPA-induced AP1 transfected in HEK293 cells assessed as inhibition of beta-lactamase reporter activity treated 1 hr before TPA stimulat... | J Nat Prod 72: 503-6 (2010) Article DOI: 10.1021/np800732n BindingDB Entry DOI: 10.7270/Q2BZ66ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor Jun (Homo sapiens (Human)) | BDBM50349194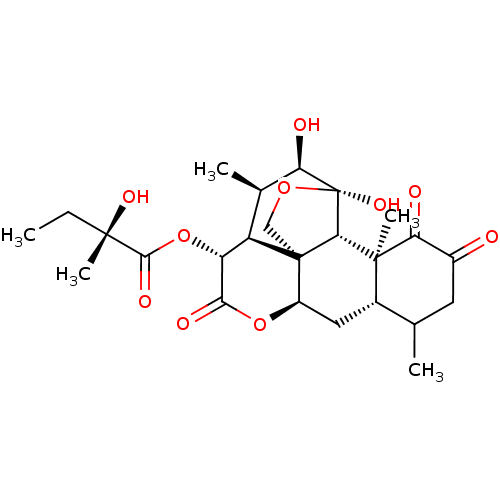 (GLAUCARUBINONE | NSC-14975) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of TPA-induced AP1 transfected in HEK293 cells assessed as inhibition of beta-lactamase reporter activity treated 1 hr before TPA stimulat... | J Nat Prod 72: 503-6 (2010) Article DOI: 10.1021/np800732n BindingDB Entry DOI: 10.7270/Q2BZ66ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50494422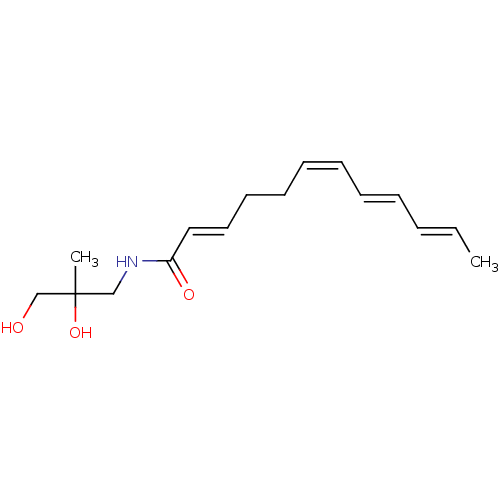 (CHEMBL3086847) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-Win55212-2 from human CB1 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after ... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50318477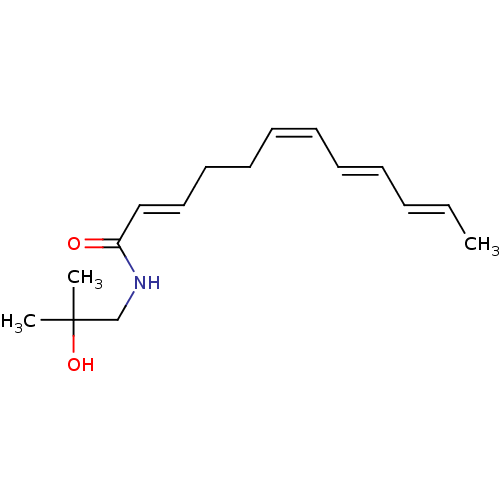 (CHEMBL1084610 | Hydroxy-alpha-sanshool) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after 1 h... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor Jun (Homo sapiens (Human)) | BDBM50295170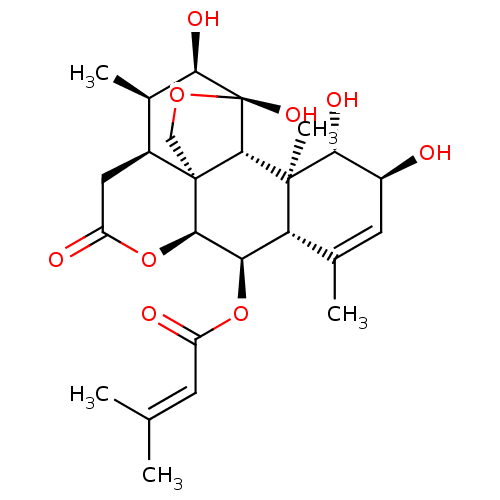 (6R-senecionylchaparrin | CHEMBL564009) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of TPA-induced AP1 transfected in HEK293 cells assessed as inhibition of beta-lactamase reporter activity treated 1 hr before TPA stimulat... | J Nat Prod 72: 503-6 (2010) Article DOI: 10.1021/np800732n BindingDB Entry DOI: 10.7270/Q2BZ66ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50494417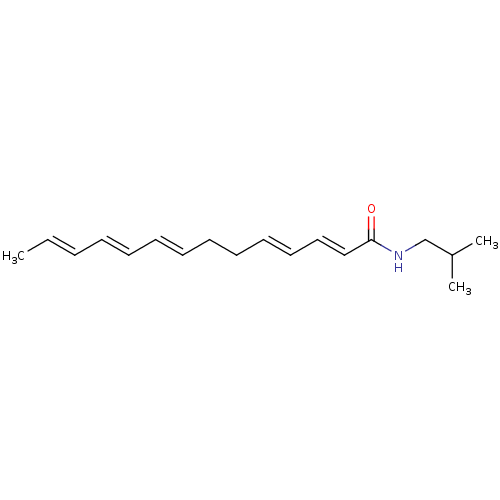 (CHEMBL3086844) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-Win55212-2 from human CB1 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after ... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50494416 (CHEMBL2335715 | Hydroxy-Beta-Sanshool) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after 1 h... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50494422 (CHEMBL3086847) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after 1 h... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50494421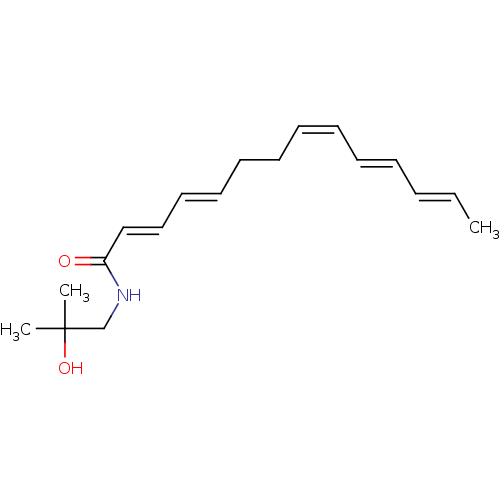 (CHEBI:81359 | CHEMBL3086846) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-Win55212-2 from human CB1 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after ... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50494421 (CHEBI:81359 | CHEMBL3086846) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after 1 h... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM33411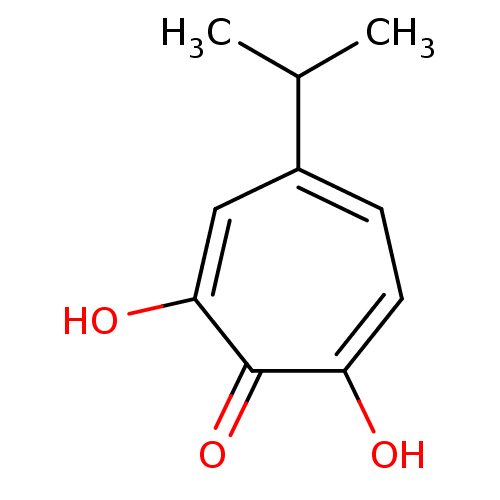 (β-Thujaplicinol | hydroxytropolone, 3) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50494420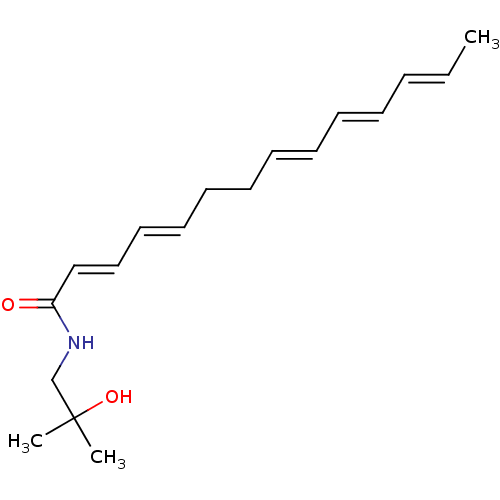 (CHEMBL3086845) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after 1 h... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484003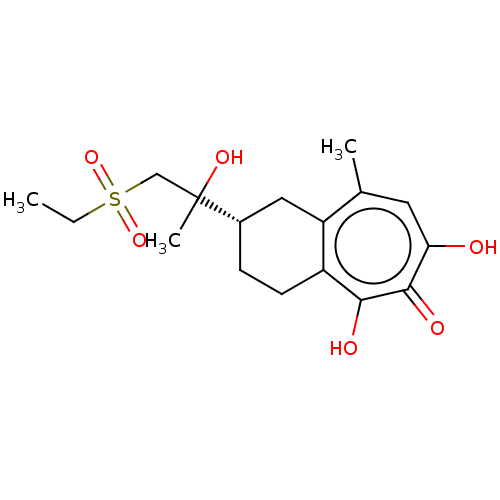 (CHEMBL1802249) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 2) | BDBM33411 (β-Thujaplicinol | hydroxytropolone, 3) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of HIV-2 reverse transcriptase | Antimicrob Agents Chemother 54: 3913-21 (2010) Article DOI: 10.1128/AAC.00434-10 BindingDB Entry DOI: 10.7270/Q2JW8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492114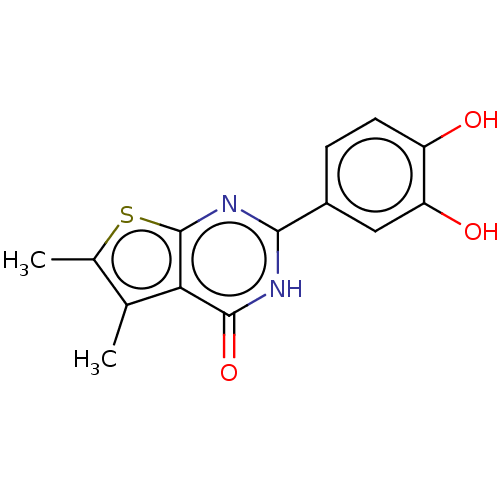 (CHEMBL2397283) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492104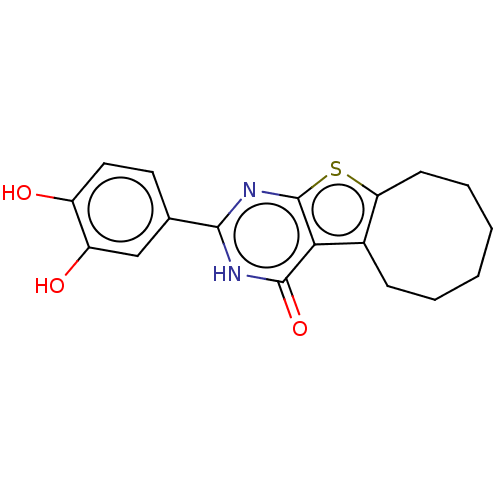 (CHEMBL2397405) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM33411 (β-Thujaplicinol | hydroxytropolone, 3) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of human immunodeficiency virus-1 reverse transcriptase associated RNase H activity using using 18-nucleotide 3,-fluorescein-labeled RNA s... | J Nat Prod 78: 315-9 (2015) Article DOI: 10.1021/np5006696 BindingDB Entry DOI: 10.7270/Q218386T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492105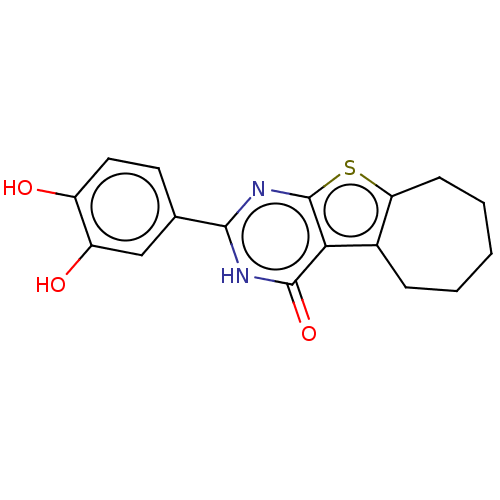 (CHEMBL2397404) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492128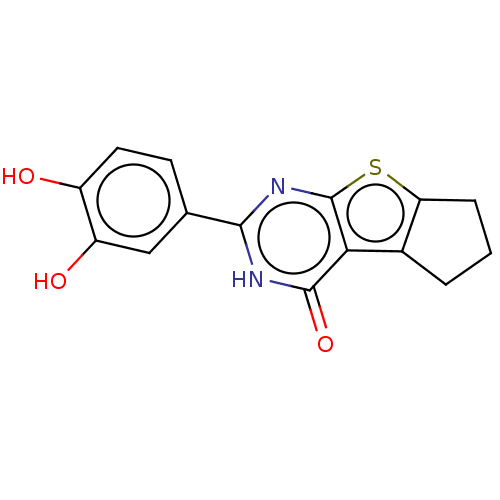 (CHEMBL2397402) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492132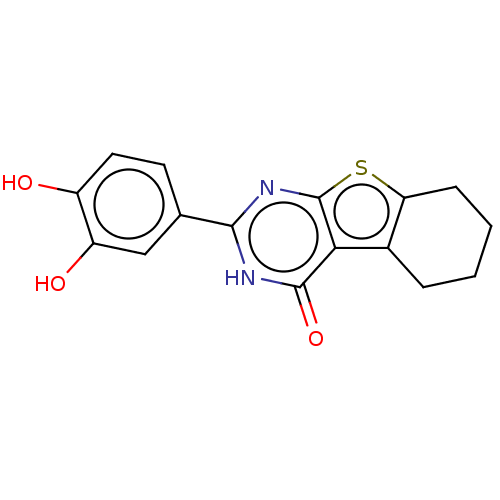 (CHEMBL2397403) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484009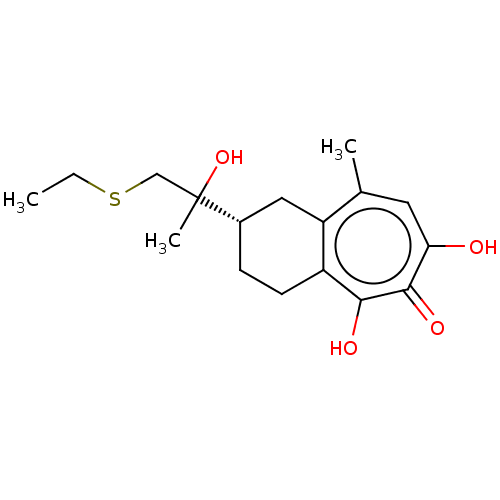 (CHEMBL1802246) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480466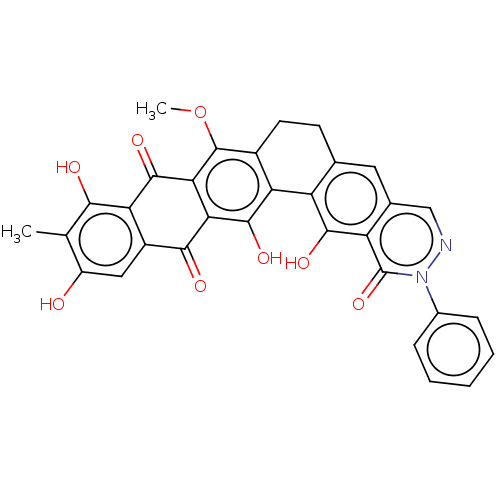 (CHEMBL541369) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492120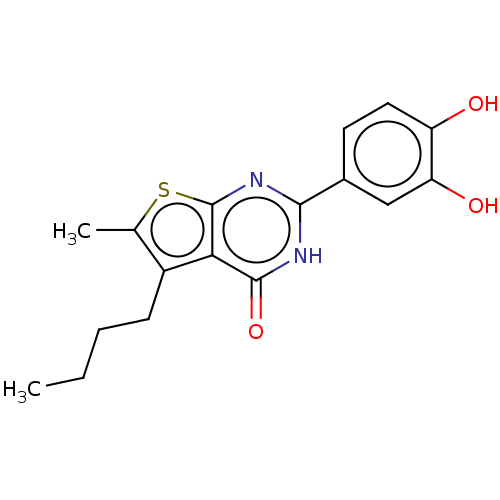 (CHEMBL2397400) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492129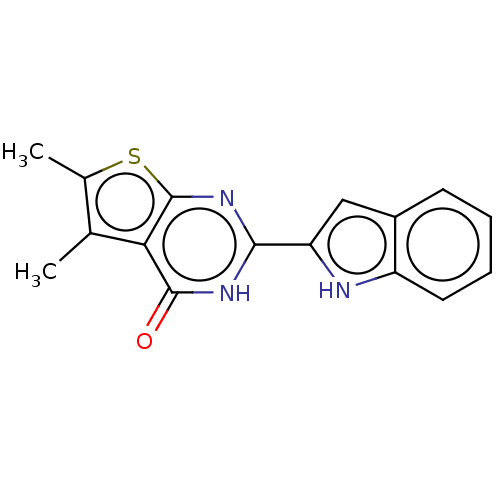 (CHEMBL2397393) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492107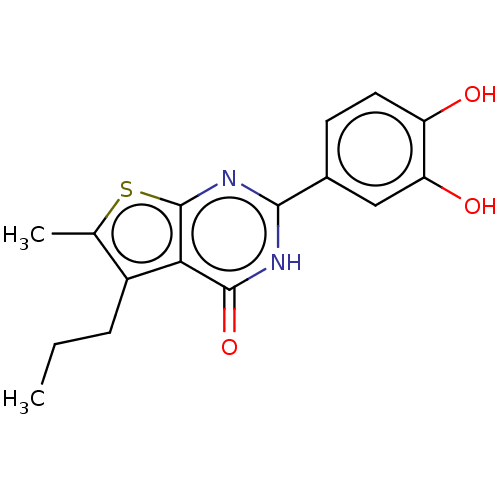 (CHEMBL2397399) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482161 (CHEMBL1082256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase-associated ribonuclease H activity by fluorescence assay | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482160 (CHEMBL1082257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase-associated ribonuclease H activity by fluorescence assay | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484014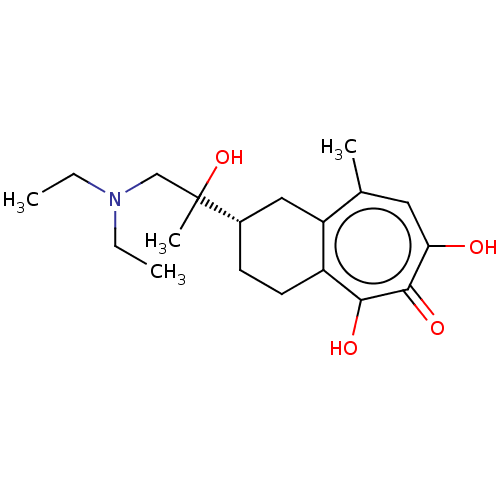 (CHEMBL1802034) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484004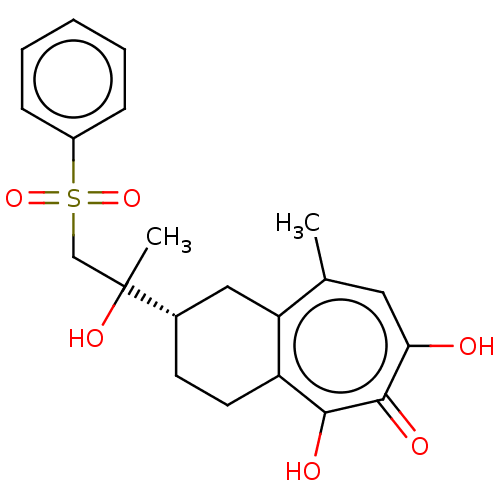 (CHEMBL1802251) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492106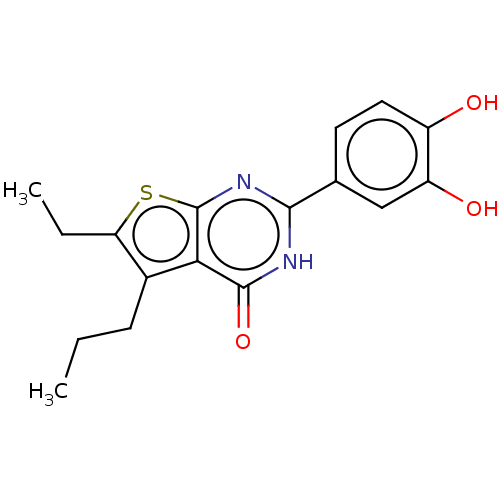 (CHEMBL2397401) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484017 (CHEMBL1802031) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50494417 (CHEMBL3086844) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 664 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells preincubated for 1 hr followed by radioligand addition measured after 1 h... | J Nat Prod 76: 2060-4 (2013) Article DOI: 10.1021/np400478c BindingDB Entry DOI: 10.7270/Q2WS8X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484015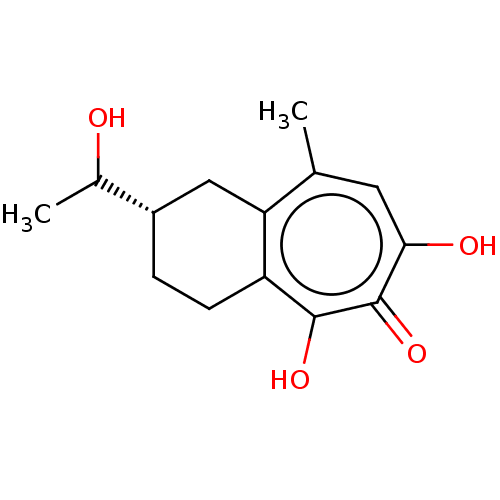 (CHEMBL1802253) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492134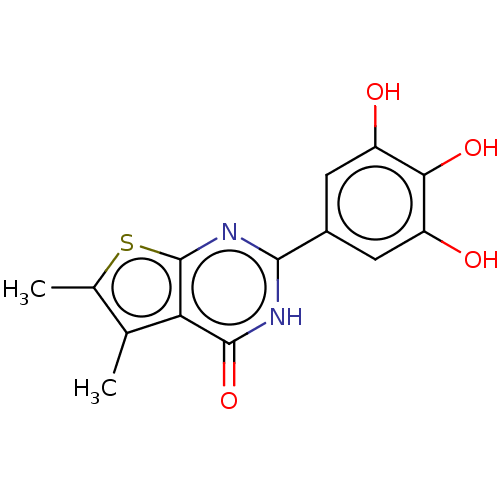 (CHEMBL2397413) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482159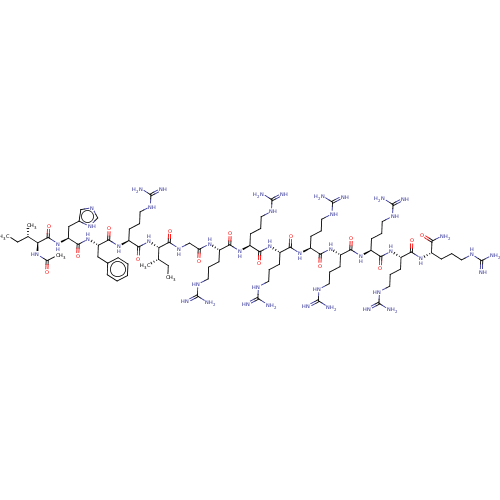 (CHEMBL1082255) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492125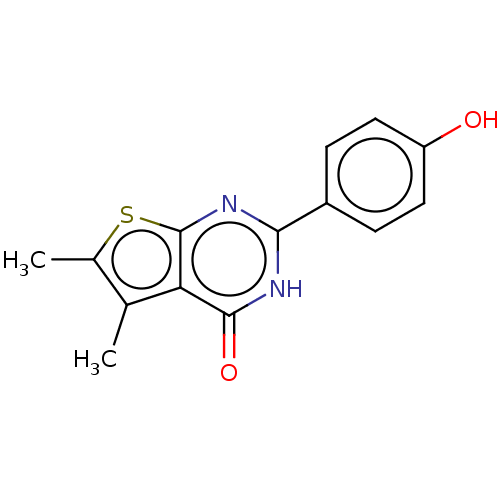 (CHEMBL2397277) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484005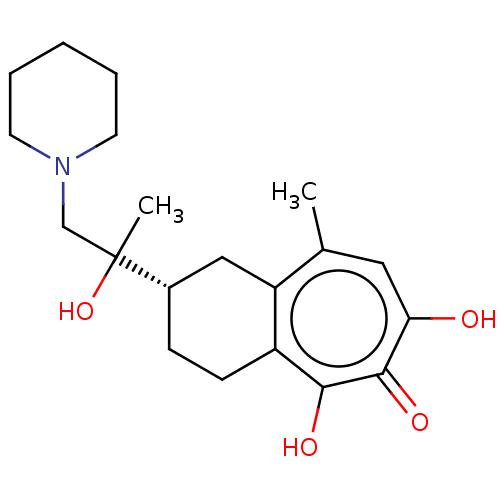 (CHEMBL1802032) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482159 (CHEMBL1082255) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-end processing activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death protein 4 (Homo sapiens (Human)) | BDBM50000298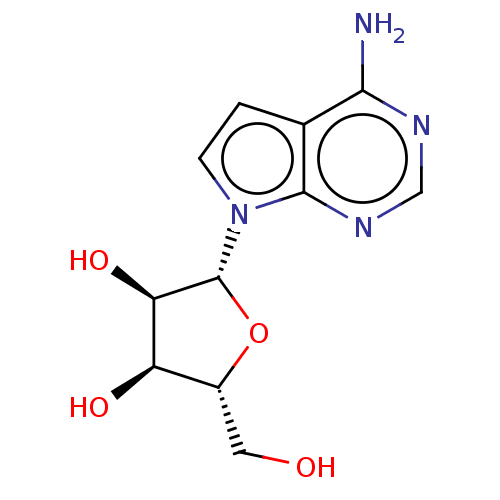 ('2-(4-AMINO-PYRROLO[2,3-D]PYRIMIDIN-7-YL)-5-HYDROX...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University Curated by ChEMBL | Assay Description Stabilization of Pdcd4 expressed in human HEK293 cells assessed as inhibition of TPA-induced degradation by luciferase reporter assay | J Nat Prod 74: 1990-5 (2011) Article DOI: 10.1021/np200603g BindingDB Entry DOI: 10.7270/Q2HQ4092 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484006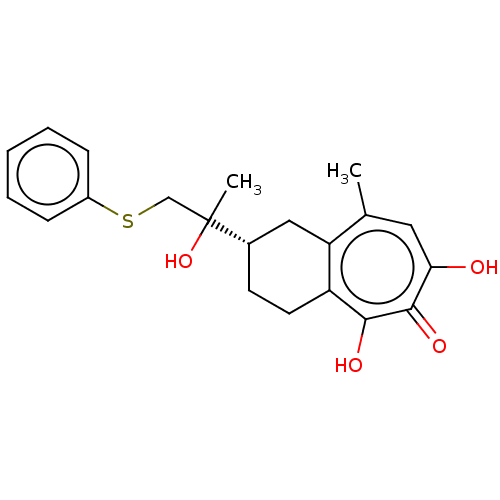 (CHEMBL1802247) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484012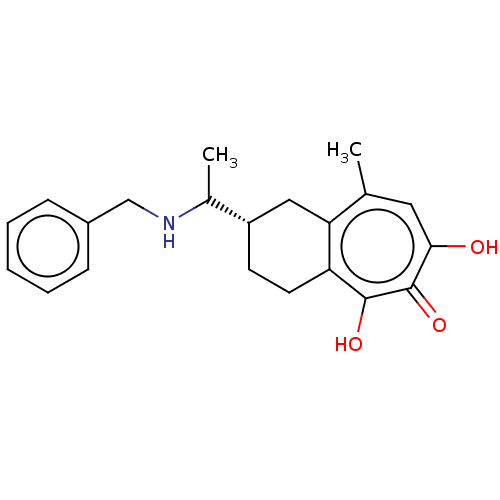 (CHEMBL1802254) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of RNase H activity of his6-tagged HIV1 HXB2 reverse transcriptase p66/p51 expressed in Escherichia coli assessed as substrate cleavage at... | J Med Chem 54: 4462-73 (2011) Article DOI: 10.1021/jm2000757 BindingDB Entry DOI: 10.7270/Q26T0QH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 167 total ) | Next | Last >> |