

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 52: 3496-504 (2009) Article DOI: 10.1021/jm9001583 BindingDB Entry DOI: 10.7270/Q2KH0N72 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of radiolabelled 1alpha, 25-(OH)2D3 from recombinant rat VDR | J Med Chem 54: 6832-42 (2011) Article DOI: 10.1021/jm200743p BindingDB Entry DOI: 10.7270/Q2K35V1M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50293284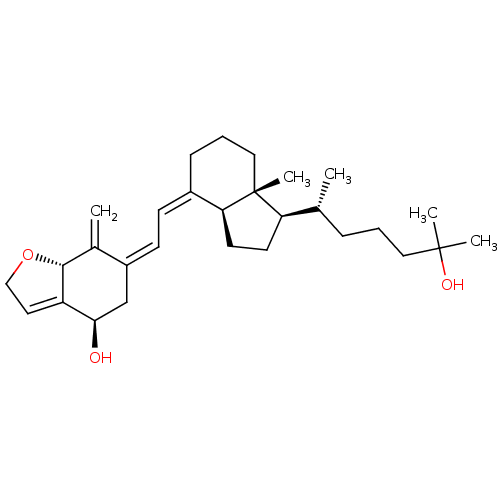 ((4R,7aR,Z)-6-((E)-2-((1R,3aS,7aR)-1-((R)-6-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 52: 3496-504 (2009) Article DOI: 10.1021/jm9001583 BindingDB Entry DOI: 10.7270/Q2KH0N72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50353860 (CHEMBL1830164) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of radiolabelled 1alpha, 25-(OH)2D3 from recombinant rat VDR | J Med Chem 54: 6832-42 (2011) Article DOI: 10.1021/jm200743p BindingDB Entry DOI: 10.7270/Q2K35V1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50353862 (CHEMBL1830166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of radiolabelled 1alpha, 25-(OH)2D3 from recombinant rat VDR | J Med Chem 54: 6832-42 (2011) Article DOI: 10.1021/jm200743p BindingDB Entry DOI: 10.7270/Q2K35V1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50353861 (CHEMBL1830165) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of radiolabelled 1alpha, 25-(OH)2D3 from recombinant rat VDR | J Med Chem 54: 6832-42 (2011) Article DOI: 10.1021/jm200743p BindingDB Entry DOI: 10.7270/Q2K35V1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50353864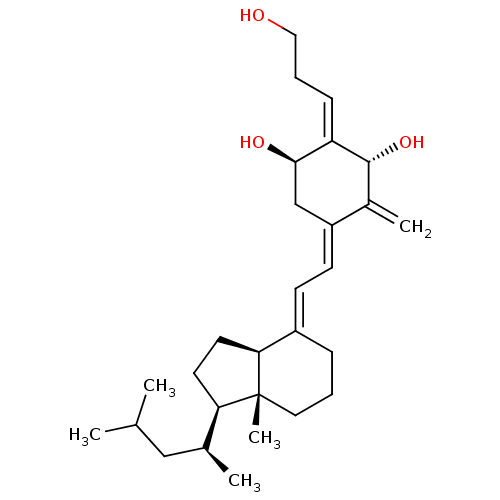 (CHEMBL1830168) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of radiolabelled 1alpha, 25-(OH)2D3 from recombinant rat VDR | J Med Chem 54: 6832-42 (2011) Article DOI: 10.1021/jm200743p BindingDB Entry DOI: 10.7270/Q2K35V1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50353863 (CHEMBL1830167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of radiolabelled 1alpha, 25-(OH)2D3 from recombinant rat VDR | J Med Chem 54: 6832-42 (2011) Article DOI: 10.1021/jm200743p BindingDB Entry DOI: 10.7270/Q2K35V1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50186661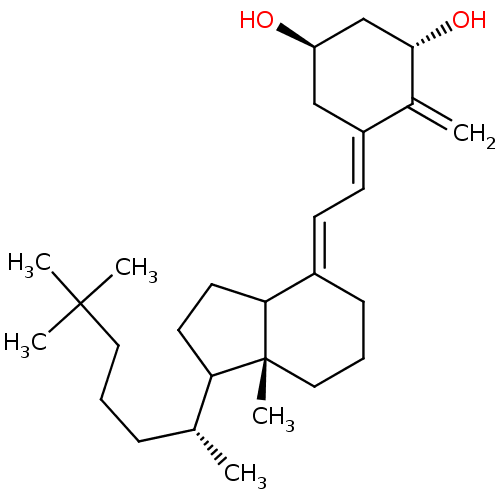 (1alpha,25-dihydroxy-2-(3'-hydroxypropylidene)-19-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha25-(OH)2D3 from rat recombinant VDR | J Med Chem 49: 2909-20 (2006) Article DOI: 10.1021/jm051082a BindingDB Entry DOI: 10.7270/Q2PZ58FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50186664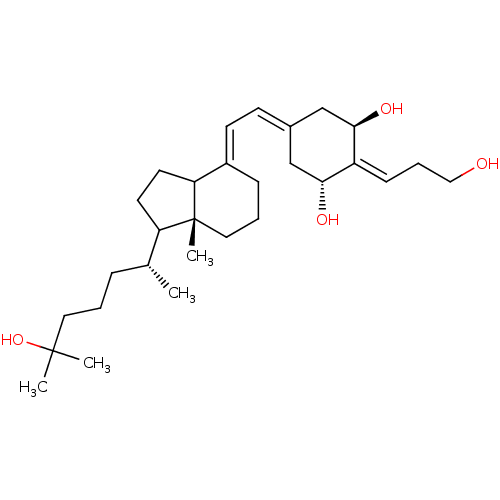 ((E)-1alpha,25-dihydroxy-2-[3''-hydroxypropylidene]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha25-(OH)2D3 from rat recombinant VDR | J Med Chem 49: 2909-20 (2006) Article DOI: 10.1021/jm051082a BindingDB Entry DOI: 10.7270/Q2PZ58FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50186662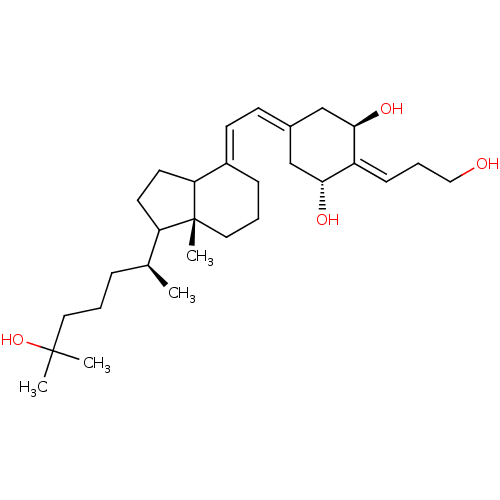 ((E)-(20S)-1alpha,25-dihydroxy-2-[3''-hydroxypropyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha25-(OH)2D3 from rat recombinant VDR | J Med Chem 49: 2909-20 (2006) Article DOI: 10.1021/jm051082a BindingDB Entry DOI: 10.7270/Q2PZ58FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50186666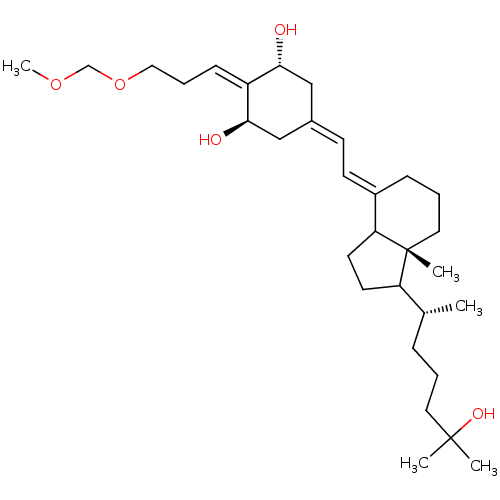 (CHEMBL207889 | E-1,25-dihydroxy-2-[3-(methoxymetho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha25-(OH)2D3 from rat recombinant VDR | J Med Chem 49: 2909-20 (2006) Article DOI: 10.1021/jm051082a BindingDB Entry DOI: 10.7270/Q2PZ58FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50293283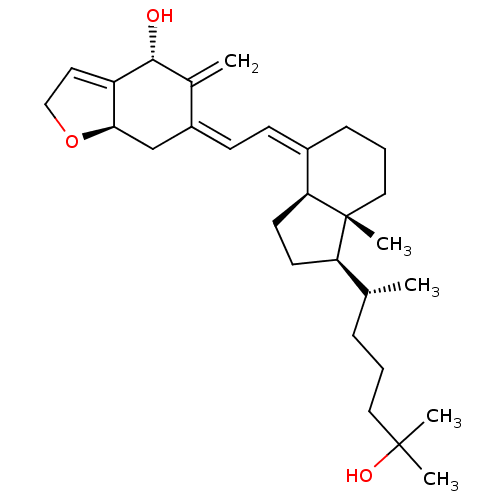 ((4R,7aR,Z)-6-((E)-2-((1R,3aS,7aR)-1-((R)-6-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 52: 3496-504 (2009) Article DOI: 10.1021/jm9001583 BindingDB Entry DOI: 10.7270/Q2KH0N72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50186664 ((E)-1alpha,25-dihydroxy-2-[3''-hydroxypropylidene]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha25-(OH)2D3 from rat recombinant VDR | J Med Chem 49: 2909-20 (2006) Article DOI: 10.1021/jm051082a BindingDB Entry DOI: 10.7270/Q2PZ58FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50186662 ((E)-(20S)-1alpha,25-dihydroxy-2-[3''-hydroxypropyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha25-(OH)2D3 from rat recombinant VDR | J Med Chem 49: 2909-20 (2006) Article DOI: 10.1021/jm051082a BindingDB Entry DOI: 10.7270/Q2PZ58FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50008072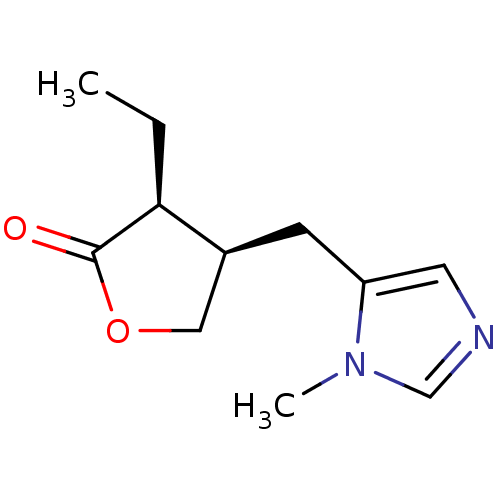 ((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 acetylcholine receptor (unknown origin) | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 acetylcholine receptor (unknown origin) | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50008072 ((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075196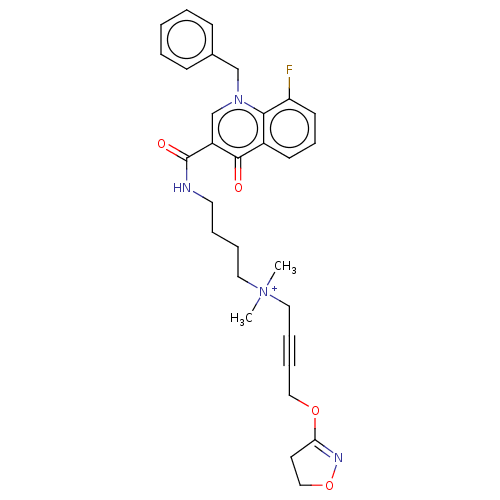 (CHEMBL3414838) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075197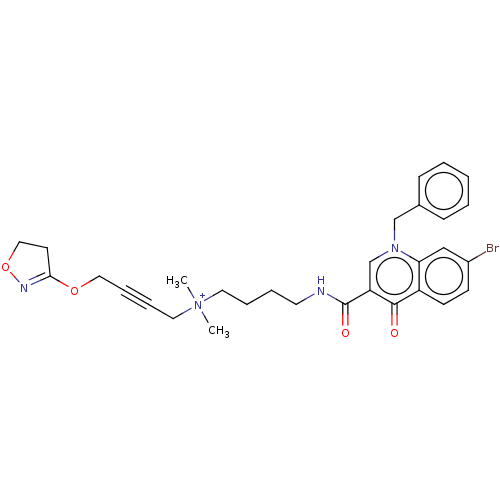 (CHEMBL3414839) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075198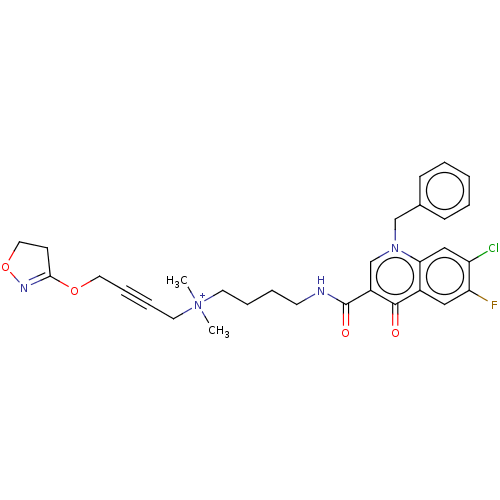 (CHEMBL3414840) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075199 (CHEMBL3414841) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075200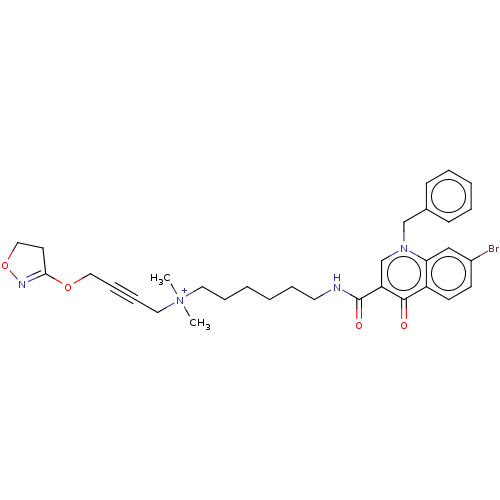 (CHEMBL3414842) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075201 (CHEMBL3414843) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 851 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075202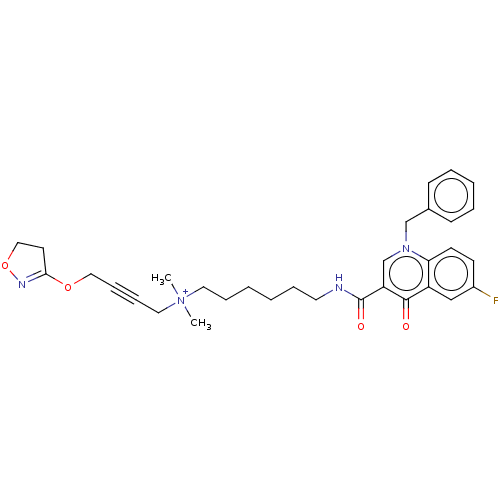 (CHEMBL3414844) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50008072 ((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 acetylcholine receptor (unknown origin) | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 acetylcholine receptor (unknown origin) | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at VDR in rat osteosarcoma cells assessed as 24-hydroxylase transcription by reporter gene assay | J Med Chem 50: 6154-64 (2007) Article DOI: 10.1021/jm070635+ BindingDB Entry DOI: 10.7270/Q2DB82PM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50292315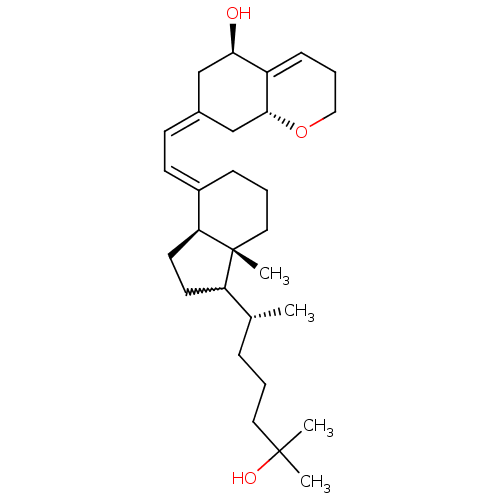 ((5R,8aR,Z)-7-((E)-2-((3aS,7aR)-1-((R)-6-hydroxy-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at VDR in rat osteosarcoma cells assessed as 24-hydroxylase transcription by reporter gene assay | J Med Chem 50: 6154-64 (2007) Article DOI: 10.1021/jm070635+ BindingDB Entry DOI: 10.7270/Q2DB82PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50292315 ((5R,8aR,Z)-7-((E)-2-((3aS,7aR)-1-((R)-6-hydroxy-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of 1-alpha,25-dihydroxyvitamin from rat recombinant VDR | J Med Chem 50: 6154-64 (2007) Article DOI: 10.1021/jm070635+ BindingDB Entry DOI: 10.7270/Q2DB82PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50292316 ((5R,8aR,E)-7-((E)-2-((3aS,7aR)-1-((R)-6-hydroxy-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of 1-alpha,25-dihydroxyvitamin from rat recombinant VDR | J Med Chem 50: 6154-64 (2007) Article DOI: 10.1021/jm070635+ BindingDB Entry DOI: 10.7270/Q2DB82PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50448377 (CHEMBL3121473) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50313638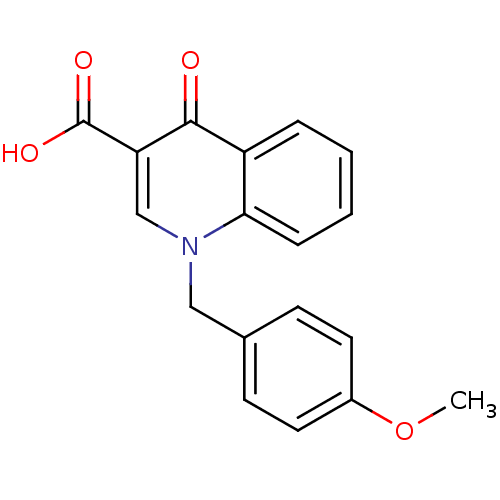 (1-(4-methoxybenzyl)-4-oxo-1,4-dihydroquinoline-3-c...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.32E+3 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075195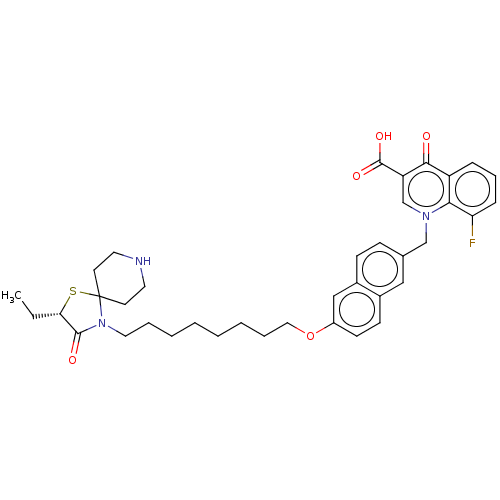 (CHEMBL3414847) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075195 (CHEMBL3414847) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075203 (CHEMBL3414845) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 537 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075202 (CHEMBL3414844) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075201 (CHEMBL3414843) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 302 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075200 (CHEMBL3414842) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075199 (CHEMBL3414841) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 178 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075198 (CHEMBL3414840) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075197 (CHEMBL3414839) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50075196 (CHEMBL3414838) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50008072 ((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 832 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50448377 (CHEMBL3121473) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of 1-alpha,25-dihydroxyvitamin from rat recombinant VDR | J Med Chem 50: 6154-64 (2007) Article DOI: 10.1021/jm070635+ BindingDB Entry DOI: 10.7270/Q2DB82PM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50313638 (1-(4-methoxybenzyl)-4-oxo-1,4-dihydroquinoline-3-c...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 acetylcholine receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 372 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||