

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466033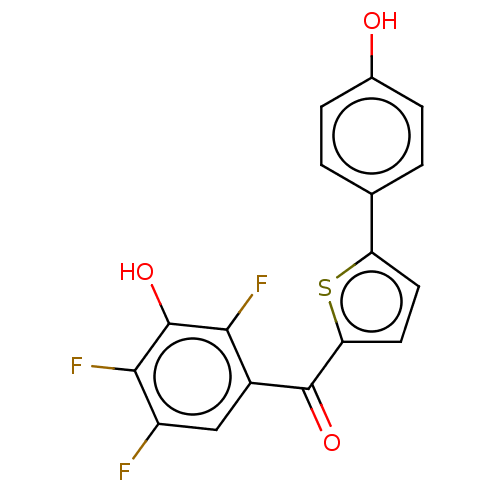 (CHEMBL4293115) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466018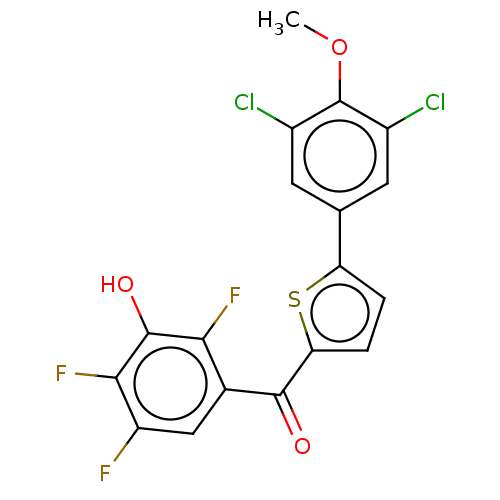 (CHEMBL4283851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466021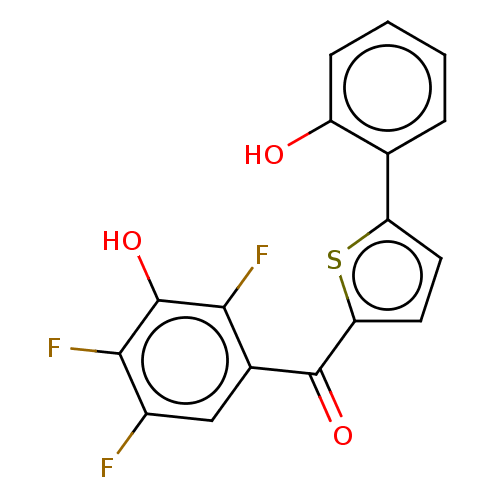 (CHEMBL4295076) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466032 (CHEMBL4276934) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466019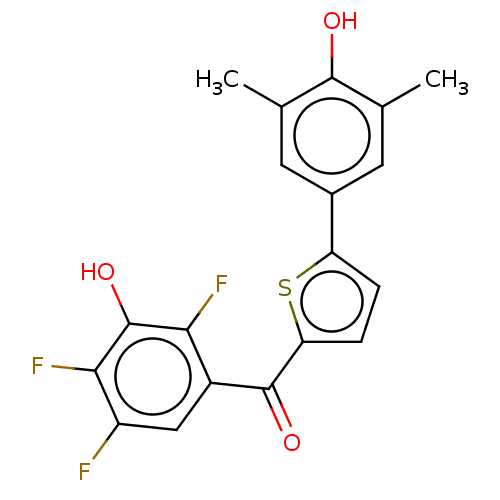 (CHEMBL4286251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239810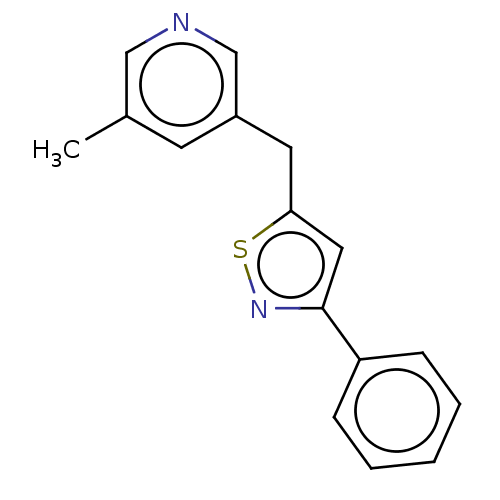 (CHEMBL4093884) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239792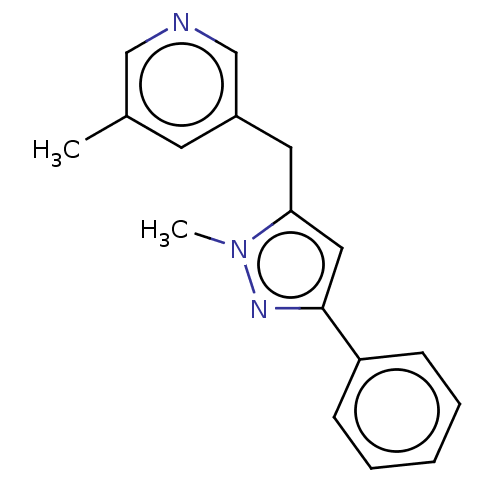 (CHEMBL4068439) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466012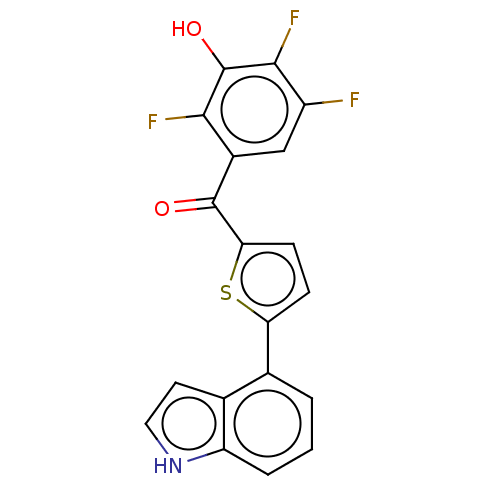 (CHEMBL4287575) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466020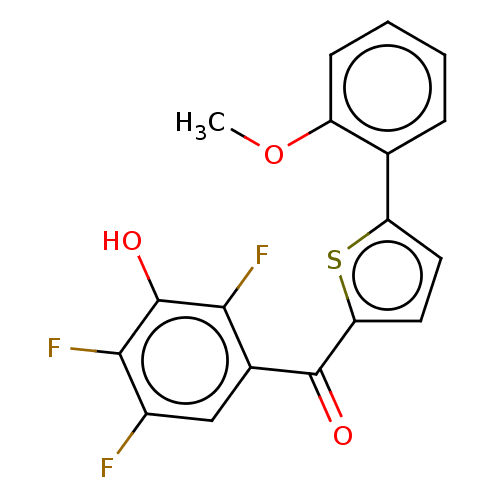 (CHEMBL4293119) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466034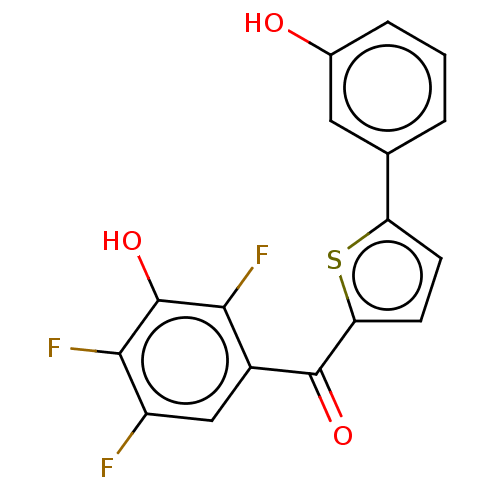 (CHEMBL4284771) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466008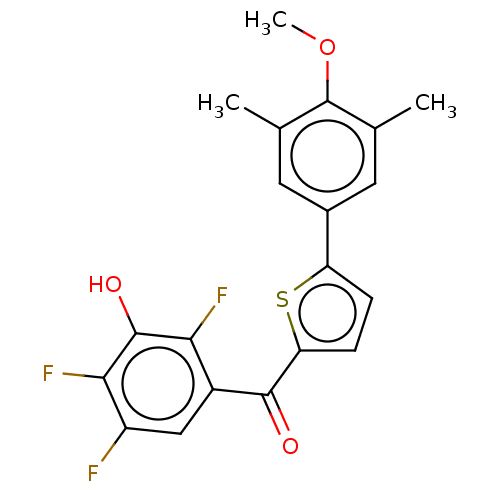 (CHEMBL4290218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466019 (CHEMBL4286251) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466027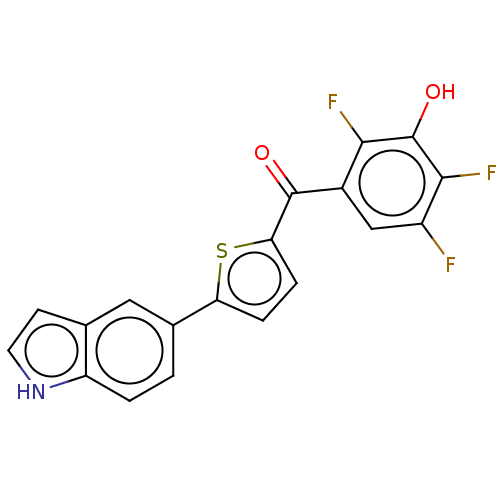 (CHEMBL4292910) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (RAT) | BDBM50318385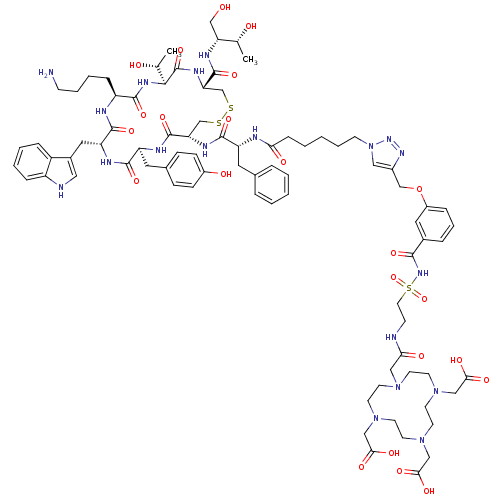 (2-(4-{[(2-{[(3-{[1-(5-{[(1R)-1-{[(4R,7S,10S,13R,16...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [111In-DOTA0,Tyr3]octreotide from SSTR2 receptor in rat AR42J cells | J Med Chem 53: 3944-53 (2010) Article DOI: 10.1021/jm100246m BindingDB Entry DOI: 10.7270/Q2PG1RX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50232146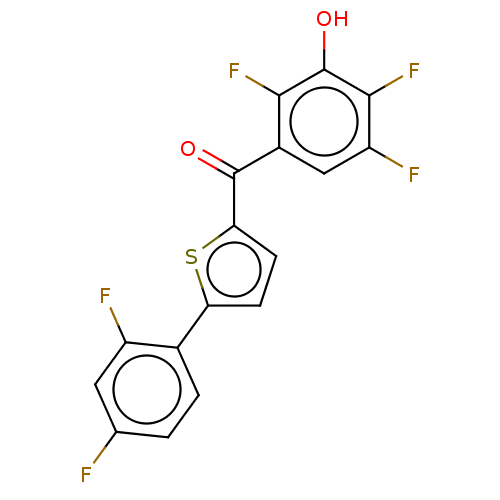 (CHEMBL4092593) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466012 (CHEMBL4287575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466018 (CHEMBL4283851) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466030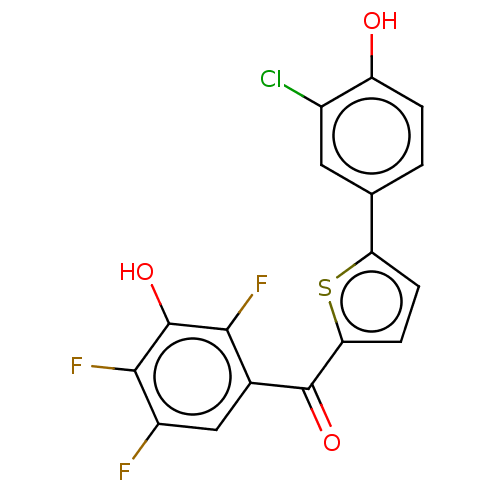 (CHEMBL4291714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466011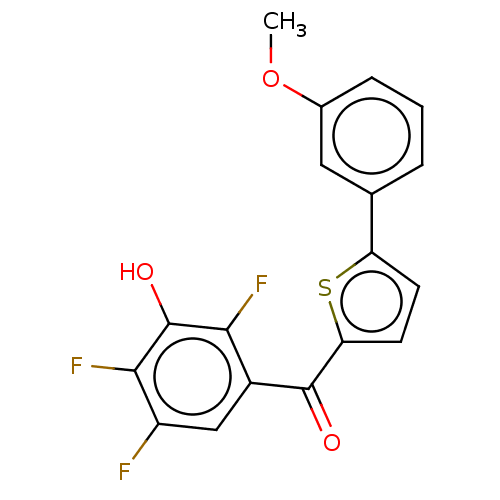 (CHEMBL4283199) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466002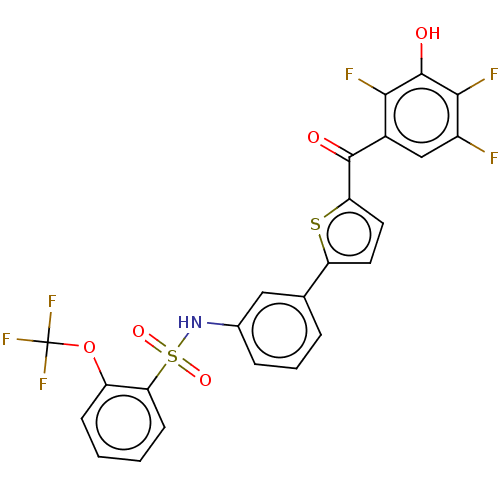 (CHEMBL4285743) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239796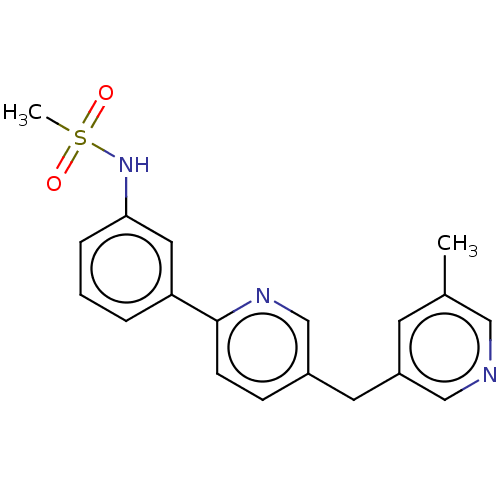 (CHEMBL4096366) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239802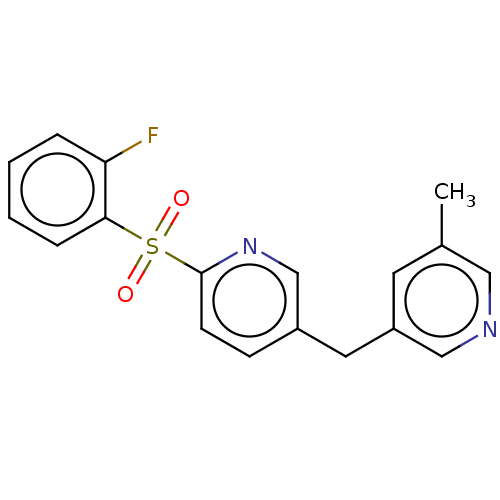 (CHEMBL4079408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239789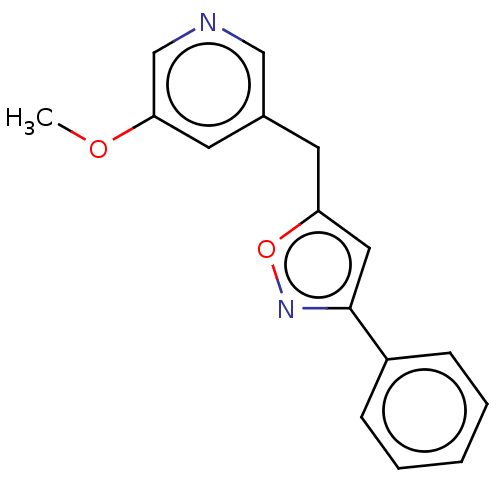 (CHEMBL4097298) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466031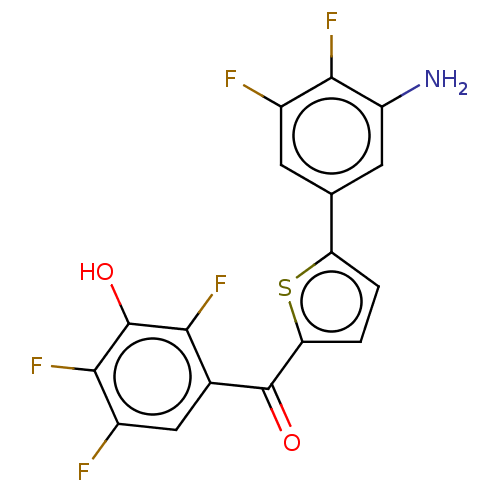 (CHEMBL4276985) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466015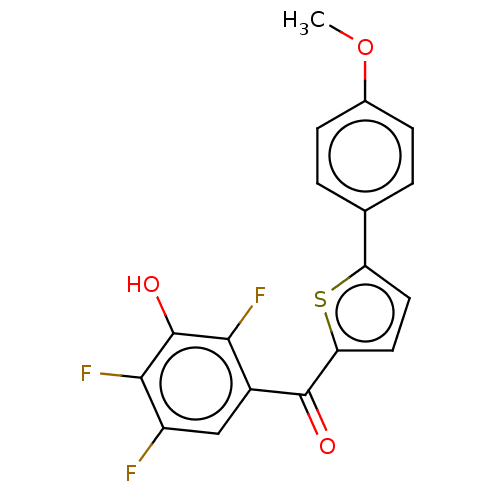 (CHEMBL4291052) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466008 (CHEMBL4290218) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (RAT) | BDBM50318386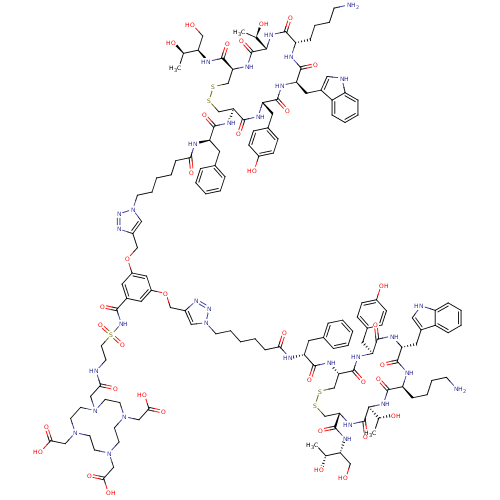 (2-[7-({[2-({[3,5-bis({[1-(5-{[(1R)-1-{[(4R,7S,10S,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [111In-DOTA0,Tyr3]octreotide from SSTR2 receptor in rat AR42J cells | J Med Chem 53: 3944-53 (2010) Article DOI: 10.1021/jm100246m BindingDB Entry DOI: 10.7270/Q2PG1RX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (RAT) | BDBM50318388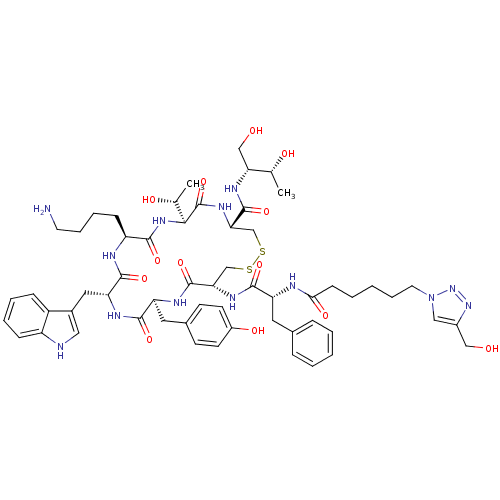 ((4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-N-[(2R,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [111In-DOTA0,Tyr3]octreotide from SSTR2 receptor in rat AR42J cells | J Med Chem 53: 3944-53 (2010) Article DOI: 10.1021/jm100246m BindingDB Entry DOI: 10.7270/Q2PG1RX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466030 (CHEMBL4291714) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466010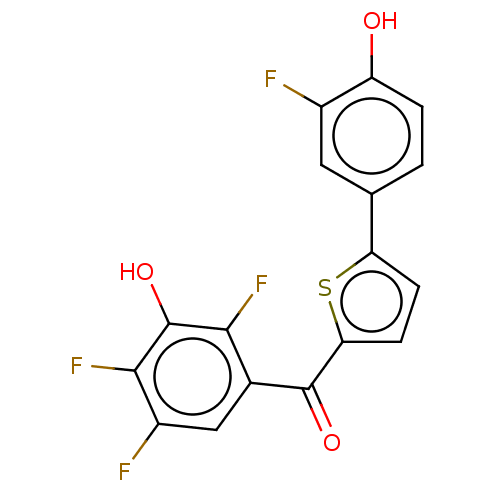 (CHEMBL4286141) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239795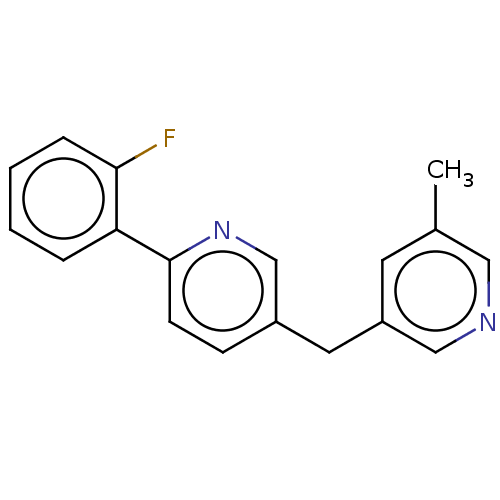 (CHEMBL4067404) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239814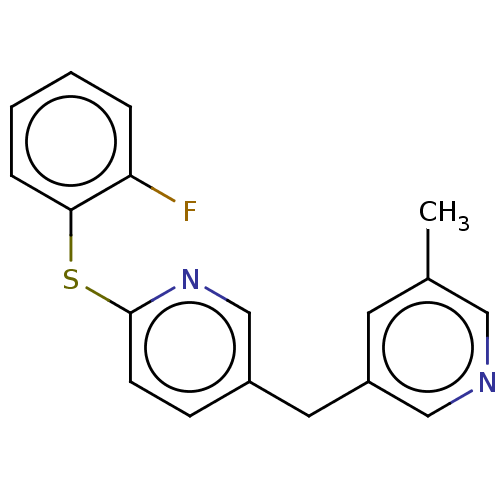 (CHEMBL4103666) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466029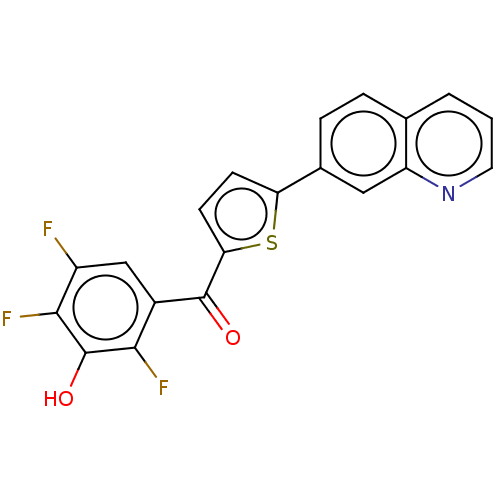 (CHEMBL4286097) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466010 (CHEMBL4286141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466034 (CHEMBL4284771) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466013 (CHEMBL4277596) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466014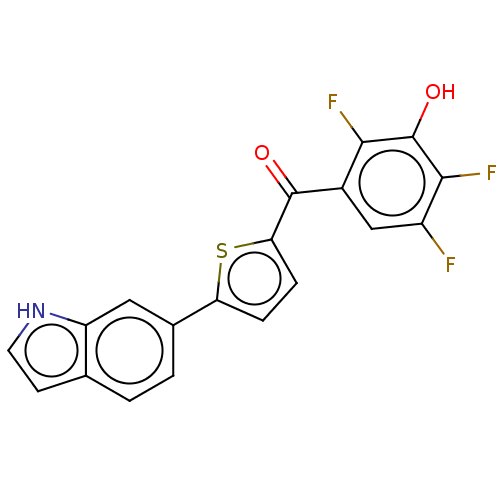 (CHEMBL4282320) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239811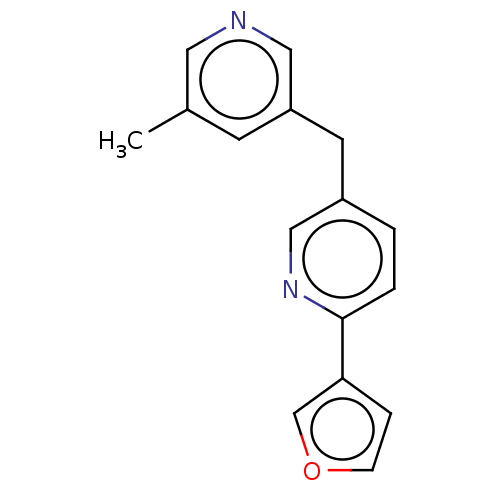 (CHEMBL4078318) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466022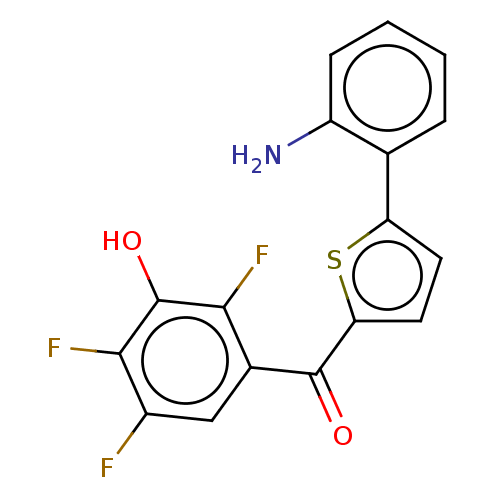 (CHEMBL4279043) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239799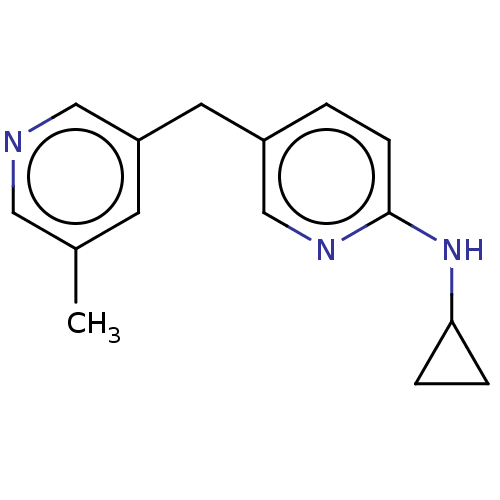 (CHEMBL4059931) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human carbonic anhydrase | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466001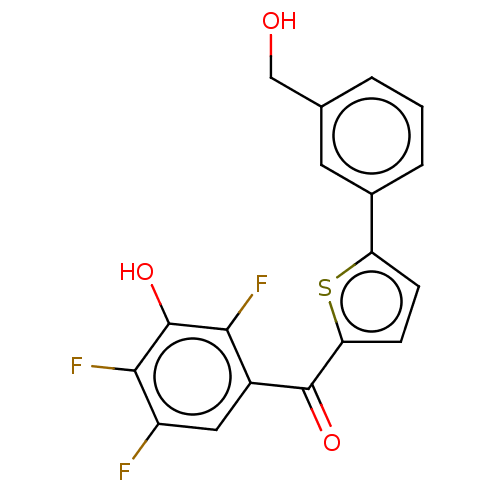 (CHEMBL4278956) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466006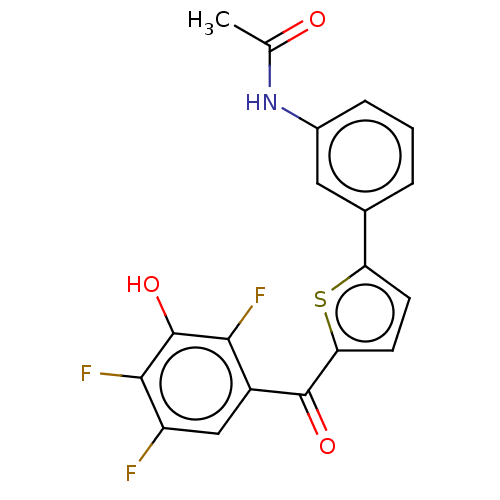 (CHEMBL4282780) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466007 (CHEMBL4289569) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239800 (CHEMBL4095910) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50239790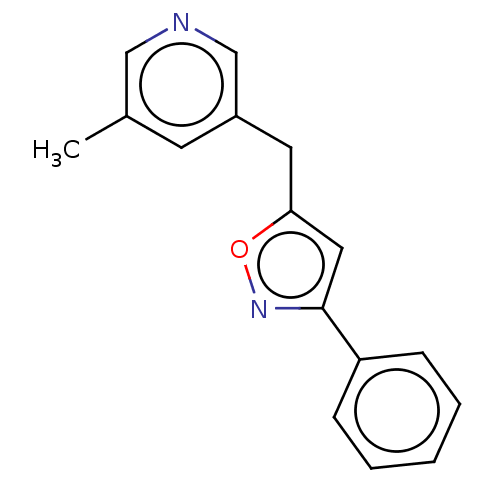 (CHEMBL4079276) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPLC analysis | J Med Chem 60: 5086-5098 (2017) Article DOI: 10.1021/acs.jmedchem.7b00437 BindingDB Entry DOI: 10.7270/Q24F1SWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466021 (CHEMBL4295076) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50466028 (CHEMBL4289526) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector bas... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50466031 (CHEMBL4276985) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmBioTec GmbH Curated by ChEMBL | Assay Description Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analys... | J Med Chem 61: 10724-10738 (2018) Article DOI: 10.1021/acs.jmedchem.8b01373 BindingDB Entry DOI: 10.7270/Q2708448 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 168 total ) | Next | Last >> |