 Found 48 hits with Last Name = 'bopp' and Initial = 's'
Found 48 hits with Last Name = 'bopp' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50128678
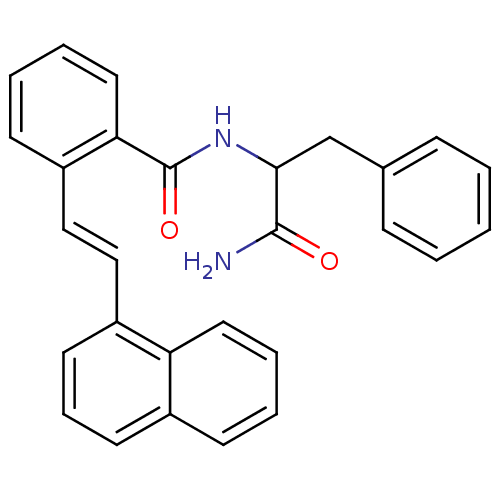
(CHEMBL310855 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-(2...)Show SMILES NC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1\C=C\c1cccc2ccccc12 Show InChI InChI=1S/C28H24N2O2/c29-27(31)26(19-20-9-2-1-3-10-20)30-28(32)25-16-7-5-12-23(25)18-17-22-14-8-13-21-11-4-6-15-24(21)22/h1-18,26H,19H2,(H2,29,31)(H,30,32)/b18-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Calpain 1 isolated from erythrocytes |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50128679
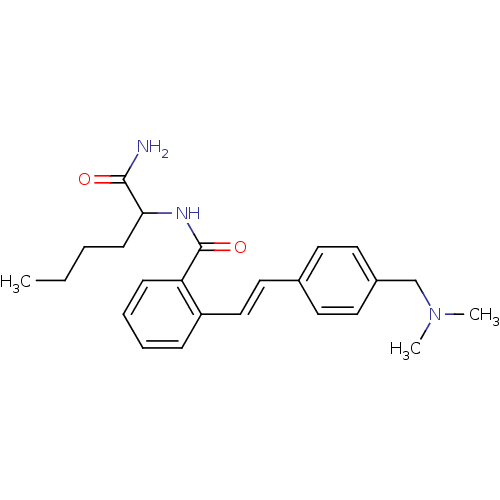
(CHEMBL77784 | N-(1-Carbamoyl-pentyl)-2-[2-(4-dimet...)Show SMILES CCCCC(NC(=O)c1ccccc1\C=C\c1ccc(CN(C)C)cc1)C(N)=O Show InChI InChI=1S/C24H31N3O2/c1-4-5-10-22(23(25)28)26-24(29)21-9-7-6-8-20(21)16-15-18-11-13-19(14-12-18)17-27(2)3/h6-9,11-16,22H,4-5,10,17H2,1-3H3,(H2,25,28)(H,26,29)/b16-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Calpain 1 isolated from erythrocytes |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50128680

(CHEMBL80903 | N-(1-Benzyl-2-oxo-ethyl)-2-(2-naphth...)Show SMILES O=CC(Cc1ccccc1)NC(=O)c1ccccc1\C=C\c1cccc2ccccc12 Show InChI InChI=1S/C28H23NO2/c30-20-25(19-21-9-2-1-3-10-21)29-28(31)27-16-7-5-12-24(27)18-17-23-14-8-13-22-11-4-6-15-26(22)23/h1-18,20,25H,19H2,(H,29,31)/b18-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Calpain 1 isolated from erythrocytes |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50073850
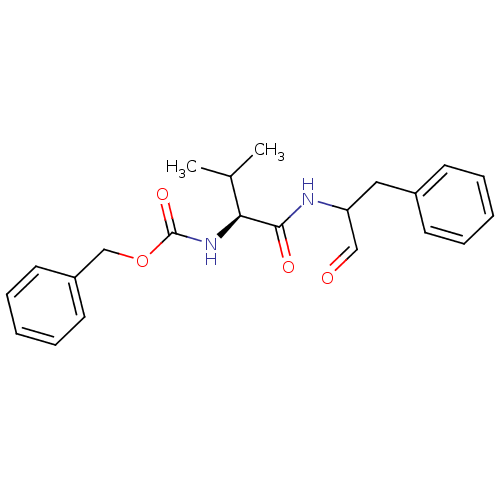
((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Calpain 1 isolated from erythrocytes |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50073850

((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50128681
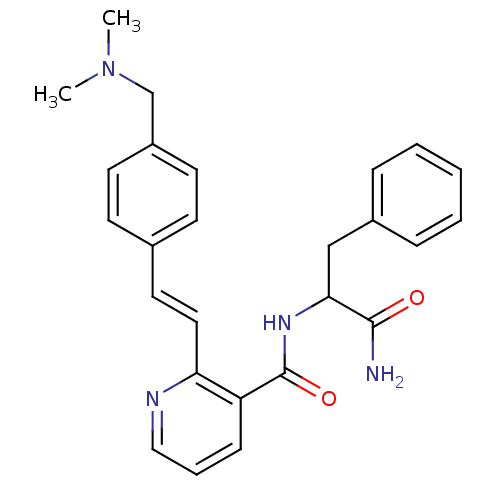
(CHEMBL80933 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...)Show SMILES CN(C)Cc1ccc(\C=C\c2ncccc2C(=O)NC(Cc2ccccc2)C(N)=O)cc1 Show InChI InChI=1S/C26H28N4O2/c1-30(2)18-21-12-10-19(11-13-21)14-15-23-22(9-6-16-28-23)26(32)29-24(25(27)31)17-20-7-4-3-5-8-20/h3-16,24H,17-18H2,1-2H3,(H2,27,31)(H,29,32)/b15-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Calpain 1 isolated from erythrocytes |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50128679

(CHEMBL77784 | N-(1-Carbamoyl-pentyl)-2-[2-(4-dimet...)Show SMILES CCCCC(NC(=O)c1ccccc1\C=C\c1ccc(CN(C)C)cc1)C(N)=O Show InChI InChI=1S/C24H31N3O2/c1-4-5-10-22(23(25)28)26-24(29)21-9-7-6-8-20(21)16-15-18-11-13-19(14-12-18)17-27(2)3/h6-9,11-16,22H,4-5,10,17H2,1-3H3,(H2,25,28)(H,26,29)/b16-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50128682
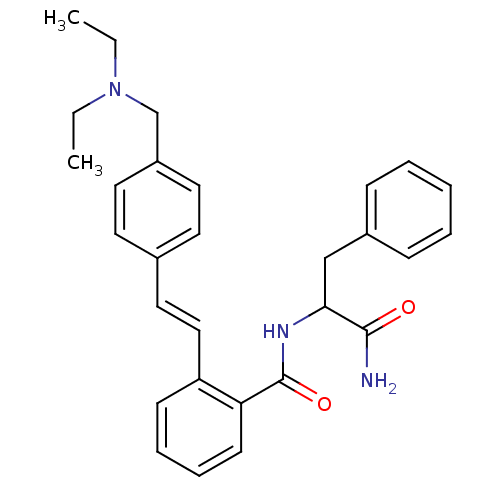
(CHEMBL80605 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...)Show SMILES CCN(CC)Cc1ccc(\C=C\c2ccccc2C(=O)NC(Cc2ccccc2)C(N)=O)cc1 Show InChI InChI=1S/C29H33N3O2/c1-3-32(4-2)21-24-16-14-22(15-17-24)18-19-25-12-8-9-13-26(25)29(34)31-27(28(30)33)20-23-10-6-5-7-11-23/h5-19,27H,3-4,20-21H2,1-2H3,(H2,30,33)(H,31,34)/b19-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Calpain 1 isolated from erythrocytes |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50128682

(CHEMBL80605 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...)Show SMILES CCN(CC)Cc1ccc(\C=C\c2ccccc2C(=O)NC(Cc2ccccc2)C(N)=O)cc1 Show InChI InChI=1S/C29H33N3O2/c1-3-32(4-2)21-24-16-14-22(15-17-24)18-19-25-12-8-9-13-26(25)29(34)31-27(28(30)33)20-23-10-6-5-7-11-23/h5-19,27H,3-4,20-21H2,1-2H3,(H2,30,33)(H,31,34)/b19-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50128681

(CHEMBL80933 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...)Show SMILES CN(C)Cc1ccc(\C=C\c2ncccc2C(=O)NC(Cc2ccccc2)C(N)=O)cc1 Show InChI InChI=1S/C26H28N4O2/c1-30(2)18-21-12-10-19(11-13-21)14-15-23-22(9-6-16-28-23)26(32)29-24(25(27)31)17-20-7-4-3-5-8-20/h3-16,24H,17-18H2,1-2H3,(H2,27,31)(H,29,32)/b15-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50128678

(CHEMBL310855 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-(2...)Show SMILES NC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1\C=C\c1cccc2ccccc12 Show InChI InChI=1S/C28H24N2O2/c29-27(31)26(19-20-9-2-1-3-10-20)30-28(32)25-16-7-5-12-23(25)18-17-22-14-8-13-21-11-4-6-15-24(21)22/h1-18,26H,19H2,(H2,29,31)(H,30,32)/b18-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50112839

(CHEMBL26631 | N-(1-Benzyl-2-oxo-ethyl)-2-styryl-be...)Show InChI InChI=1S/C24H21NO2/c26-18-22(17-20-11-5-2-6-12-20)25-24(27)23-14-8-7-13-21(23)16-15-19-9-3-1-4-10-19/h1-16,18,22H,17H2,(H,25,27)/b16-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Calpain 1 isolated from erythrocytes |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50112838

(CHEMBL281874 | N-(1-Benzyl-2-oxo-ethyl)-benzamide)Show InChI InChI=1S/C16H15NO2/c18-12-15(11-13-7-3-1-4-8-13)17-16(19)14-9-5-2-6-10-14/h1-10,12,15H,11H2,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Calpain 1 isolated from erythrocytes |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50022775

((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...)Show InChI InChI=1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50022775

((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...)Show InChI InChI=1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50022775

((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...)Show InChI InChI=1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50298426
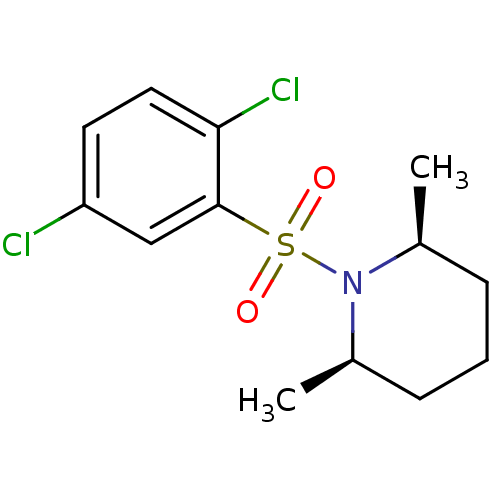
(1-(2,5-dichloro benzene)-sulfonyl-cis-2,6-dimethyl...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C13H17Cl2NO2S/c1-9-4-3-5-10(2)16(9)19(17,18)13-8-11(14)6-7-12(13)15/h6-10H,3-5H2,1-2H3/t9-,10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298421

(CHEMBL576005 | cis-2,6-dimethyl-1-methyl sulfonyl ...)Show InChI InChI=1S/C8H17NO2S/c1-7-5-4-6-8(2)9(7)12(3,10)11/h7-8H,4-6H2,1-3H3/t7-,8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50298426

(1-(2,5-dichloro benzene)-sulfonyl-cis-2,6-dimethyl...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C13H17Cl2NO2S/c1-9-4-3-5-10(2)16(9)19(17,18)13-8-11(14)6-7-12(13)15/h6-10H,3-5H2,1-2H3/t9-,10+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50298421

(CHEMBL576005 | cis-2,6-dimethyl-1-methyl sulfonyl ...)Show InChI InChI=1S/C8H17NO2S/c1-7-5-4-6-8(2)9(7)12(3,10)11/h7-8H,4-6H2,1-3H3/t7-,8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50298421

(CHEMBL576005 | cis-2,6-dimethyl-1-methyl sulfonyl ...)Show InChI InChI=1S/C8H17NO2S/c1-7-5-4-6-8(2)9(7)12(3,10)11/h7-8H,4-6H2,1-3H3/t7-,8+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298426

(1-(2,5-dichloro benzene)-sulfonyl-cis-2,6-dimethyl...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C13H17Cl2NO2S/c1-9-4-3-5-10(2)16(9)19(17,18)13-8-11(14)6-7-12(13)15/h6-10H,3-5H2,1-2H3/t9-,10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50298427

(1-(2-nitro benzene)-sulfonyl-cis-2,6-dimethylpiper...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccccc1[N+]([O-])=O |r| Show InChI InChI=1S/C13H18N2O4S/c1-10-6-5-7-11(2)14(10)20(18,19)13-9-4-3-8-12(13)15(16)17/h3-4,8-11H,5-7H2,1-2H3/t10-,11+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298427

(1-(2-nitro benzene)-sulfonyl-cis-2,6-dimethylpiper...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccccc1[N+]([O-])=O |r| Show InChI InChI=1S/C13H18N2O4S/c1-10-6-5-7-11(2)14(10)20(18,19)13-9-4-3-8-12(13)15(16)17/h3-4,8-11H,5-7H2,1-2H3/t10-,11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50298428

(1-(3-nitro benzene)-sulfonyl-cis-2,6-dimethylpiper...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1cccc(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C13H18N2O4S/c1-10-5-3-6-11(2)14(10)20(18,19)13-8-4-7-12(9-13)15(16)17/h4,7-11H,3,5-6H2,1-2H3/t10-,11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298428

(1-(3-nitro benzene)-sulfonyl-cis-2,6-dimethylpiper...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1cccc(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C13H18N2O4S/c1-10-5-3-6-11(2)14(10)20(18,19)13-8-4-7-12(9-13)15(16)17/h4,7-11H,3,5-6H2,1-2H3/t10-,11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50298427

(1-(2-nitro benzene)-sulfonyl-cis-2,6-dimethylpiper...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccccc1[N+]([O-])=O |r| Show InChI InChI=1S/C13H18N2O4S/c1-10-6-5-7-11(2)14(10)20(18,19)13-9-4-3-8-12(13)15(16)17/h3-4,8-11H,5-7H2,1-2H3/t10-,11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50298428

(1-(3-nitro benzene)-sulfonyl-cis-2,6-dimethylpiper...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1cccc(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C13H18N2O4S/c1-10-5-3-6-11(2)14(10)20(18,19)13-8-4-7-12(9-13)15(16)17/h4,7-11H,3,5-6H2,1-2H3/t10-,11+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50298425
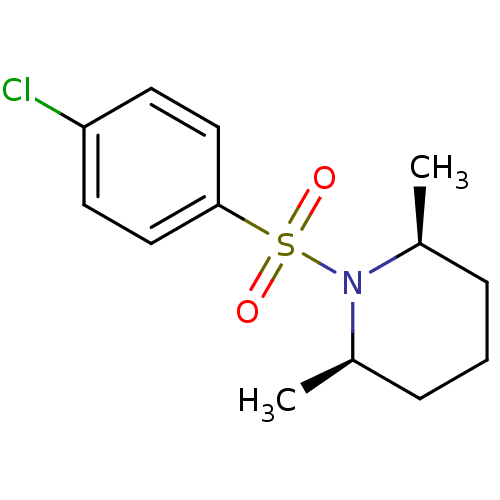
((2R,6S)-1-(4-chlorophenylsulfonyl)-2,6-dimethylpip...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C13H18ClNO2S/c1-10-4-3-5-11(2)15(10)18(16,17)13-8-6-12(14)7-9-13/h6-11H,3-5H2,1-2H3/t10-,11+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298425

((2R,6S)-1-(4-chlorophenylsulfonyl)-2,6-dimethylpip...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C13H18ClNO2S/c1-10-4-3-5-11(2)15(10)18(16,17)13-8-6-12(14)7-9-13/h6-11H,3-5H2,1-2H3/t10-,11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 318 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50298425

((2R,6S)-1-(4-chlorophenylsulfonyl)-2,6-dimethylpip...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C13H18ClNO2S/c1-10-4-3-5-11(2)15(10)18(16,17)13-8-6-12(14)7-9-13/h6-11H,3-5H2,1-2H3/t10-,11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50298423

(1-(4-methyl benzene)-sulfonyl-cis-2,6-dimethylpipe...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C14H21NO2S/c1-11-7-9-14(10-8-11)18(16,17)15-12(2)5-4-6-13(15)3/h7-10,12-13H,4-6H2,1-3H3/t12-,13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50298422
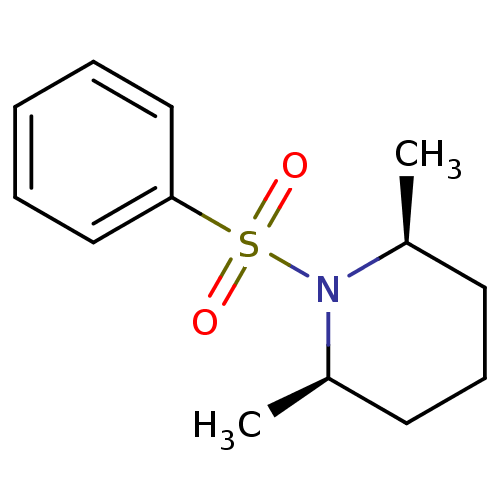
(1-benzene sulfonyl-cis-2,6-dimethyl piperidine | C...)Show InChI InChI=1S/C13H19NO2S/c1-11-7-6-8-12(2)14(11)17(15,16)13-9-4-3-5-10-13/h3-5,9-12H,6-8H2,1-2H3/t11-,12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50298423

(1-(4-methyl benzene)-sulfonyl-cis-2,6-dimethylpipe...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C14H21NO2S/c1-11-7-9-14(10-8-11)18(16,17)15-12(2)5-4-6-13(15)3/h7-10,12-13H,4-6H2,1-3H3/t12-,13+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298423

(1-(4-methyl benzene)-sulfonyl-cis-2,6-dimethylpipe...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C14H21NO2S/c1-11-7-9-14(10-8-11)18(16,17)15-12(2)5-4-6-13(15)3/h7-10,12-13H,4-6H2,1-3H3/t12-,13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50298422

(1-benzene sulfonyl-cis-2,6-dimethyl piperidine | C...)Show InChI InChI=1S/C13H19NO2S/c1-11-7-6-8-12(2)14(11)17(15,16)13-9-4-3-5-10-13/h3-5,9-12H,6-8H2,1-2H3/t11-,12+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298422

(1-benzene sulfonyl-cis-2,6-dimethyl piperidine | C...)Show InChI InChI=1S/C13H19NO2S/c1-11-7-6-8-12(2)14(11)17(15,16)13-9-4-3-5-10-13/h3-5,9-12H,6-8H2,1-2H3/t11-,12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50298424
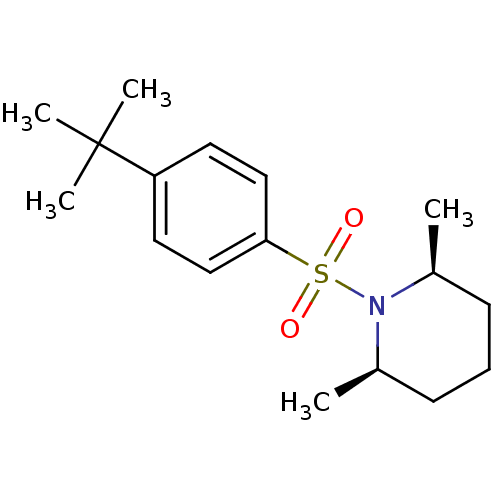
(1-(4-tert-butyl benzene)-sulfonyl-cis-2,6-dimethyl...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C17H27NO2S/c1-13-7-6-8-14(2)18(13)21(19,20)16-11-9-15(10-12-16)17(3,4)5/h9-14H,6-8H2,1-5H3/t13-,14+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298424

(1-(4-tert-butyl benzene)-sulfonyl-cis-2,6-dimethyl...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C17H27NO2S/c1-13-7-6-8-14(2)18(13)21(19,20)16-11-9-15(10-12-16)17(3,4)5/h9-14H,6-8H2,1-5H3/t13-,14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 458 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50298424

(1-(4-tert-butyl benzene)-sulfonyl-cis-2,6-dimethyl...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C17H27NO2S/c1-13-7-6-8-14(2)18(13)21(19,20)16-11-9-15(10-12-16)17(3,4)5/h9-14H,6-8H2,1-5H3/t13-,14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50073850

((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of degradation of tyrosine kinase pp60src by Calpain 1 in human platelets |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50128682

(CHEMBL80605 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...)Show SMILES CCN(CC)Cc1ccc(\C=C\c2ccccc2C(=O)NC(Cc2ccccc2)C(N)=O)cc1 Show InChI InChI=1S/C29H33N3O2/c1-3-32(4-2)21-24-16-14-22(15-17-24)18-19-25-12-8-9-13-26(25)29(34)31-27(28(30)33)20-23-10-6-5-7-11-23/h5-19,27H,3-4,20-21H2,1-2H3,(H2,30,33)(H,31,34)/b19-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of degradation of tyrosine kinase pp60src by Calpain 1 in human platelets |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50128679

(CHEMBL77784 | N-(1-Carbamoyl-pentyl)-2-[2-(4-dimet...)Show SMILES CCCCC(NC(=O)c1ccccc1\C=C\c1ccc(CN(C)C)cc1)C(N)=O Show InChI InChI=1S/C24H31N3O2/c1-4-5-10-22(23(25)28)26-24(29)21-9-7-6-8-20(21)16-15-18-11-13-19(14-12-18)17-27(2)3/h6-9,11-16,22H,4-5,10,17H2,1-3H3,(H2,25,28)(H,26,29)/b16-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of degradation of tyrosine kinase pp60src by Calpain 1 in human platelets |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50128681

(CHEMBL80933 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...)Show SMILES CN(C)Cc1ccc(\C=C\c2ncccc2C(=O)NC(Cc2ccccc2)C(N)=O)cc1 Show InChI InChI=1S/C26H28N4O2/c1-30(2)18-21-12-10-19(11-13-21)14-15-23-22(9-6-16-28-23)26(32)29-24(25(27)31)17-20-7-4-3-5-8-20/h3-16,24H,17-18H2,1-2H3,(H2,27,31)(H,29,32)/b15-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of degradation of tyrosine kinase pp60src by Calpain 1 in human platelets |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298429

(1-(4-nitro benzene)-sulfonyl-cis-2,6-dimethylpiper...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C13H18N2O4S/c1-10-4-3-5-11(2)14(10)20(18,19)13-8-6-12(7-9-13)15(16)17/h6-11H,3-5H2,1-2H3/t10-,11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of human serum AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50298429

(1-(4-nitro benzene)-sulfonyl-cis-2,6-dimethylpiper...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C13H18N2O4S/c1-10-4-3-5-11(2)14(10)20(18,19)13-8-6-12(7-9-13)15(16)17/h6-11H,3-5H2,1-2H3/t10-,11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat brain homogenate AChE by Ellman's assay |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50298429

(1-(4-nitro benzene)-sulfonyl-cis-2,6-dimethylpiper...)Show SMILES C[C@H]1CCC[C@@H](C)N1S(=O)(=O)c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C13H18N2O4S/c1-10-4-3-5-11(2)14(10)20(18,19)13-8-6-12(7-9-13)15(16)17/h6-11H,3-5H2,1-2H3/t10-,11+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 4057-62 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.042
BindingDB Entry DOI: 10.7270/Q2MK6CZF |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50128678

(CHEMBL310855 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-(2...)Show SMILES NC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1\C=C\c1cccc2ccccc12 Show InChI InChI=1S/C28H24N2O2/c29-27(31)26(19-20-9-2-1-3-10-20)30-28(32)25-16-7-5-12-23(25)18-17-22-14-8-13-21-11-4-6-15-24(21)22/h1-18,26H,19H2,(H2,29,31)(H,30,32)/b18-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of degradation of tyrosine kinase pp60src by Calpain 1 in human platelets |
J Med Chem 46: 2404-12 (2003)
Article DOI: 10.1021/jm0210717
BindingDB Entry DOI: 10.7270/Q28P5ZVP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data