

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galanin receptor type 3 (Homo sapiens (Human)) | BDBM50187923 (1,3-dihydro-1-phenyl-3-[[3-(trilfluoromethyl)pheny...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]galanin from human GAL3 | J Med Chem 49: 3757-8 (2006) Article DOI: 10.1021/jm060001n BindingDB Entry DOI: 10.7270/Q2T72H2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 3 (Homo sapiens (Human)) | BDBM50189598 (1-(ethylpropyl)-3-{[3-(trifluoromethyl)phenyl]azam...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]galanin from human GAL3 | J Med Chem 49: 3757-8 (2006) Article DOI: 10.1021/jm060001n BindingDB Entry DOI: 10.7270/Q2T72H2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50187923 (1,3-dihydro-1-phenyl-3-[[3-(trilfluoromethyl)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT4 | J Med Chem 49: 3757-8 (2006) Article DOI: 10.1021/jm060001n BindingDB Entry DOI: 10.7270/Q2T72H2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 3 (Homo sapiens (Human)) | BDBM50189596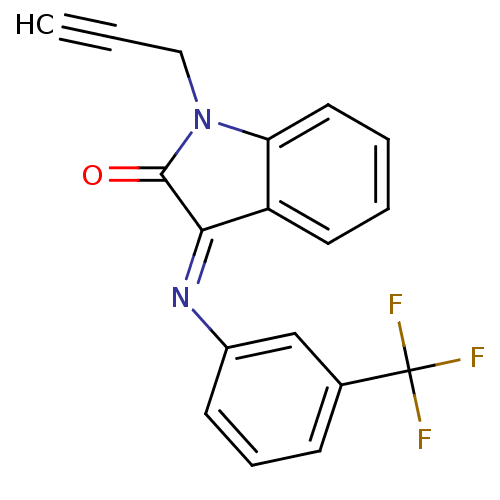 (1-(2-propynyl)-3-{[3-(trifluoromethyl)phenyl]azame...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]galanin from human GAL3 | J Med Chem 49: 3757-8 (2006) Article DOI: 10.1021/jm060001n BindingDB Entry DOI: 10.7270/Q2T72H2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 3 (Homo sapiens (Human)) | BDBM50189600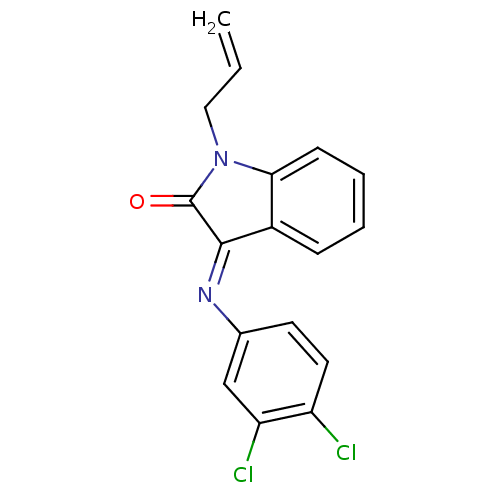 ((E)-1-allyl-3-(3,4-dichlorophenylimino)indolin-2-o...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]galanin from human GAL3 | J Med Chem 49: 3757-8 (2006) Article DOI: 10.1021/jm060001n BindingDB Entry DOI: 10.7270/Q2T72H2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 3 (Homo sapiens (Human)) | BDBM50189597 ((E)-3-(2,3-dichlorophenylimino)indolin-2-one | CHE...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]galanin from human GAL3 | J Med Chem 49: 3757-8 (2006) Article DOI: 10.1021/jm060001n BindingDB Entry DOI: 10.7270/Q2T72H2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 3 (Homo sapiens (Human)) | BDBM50189595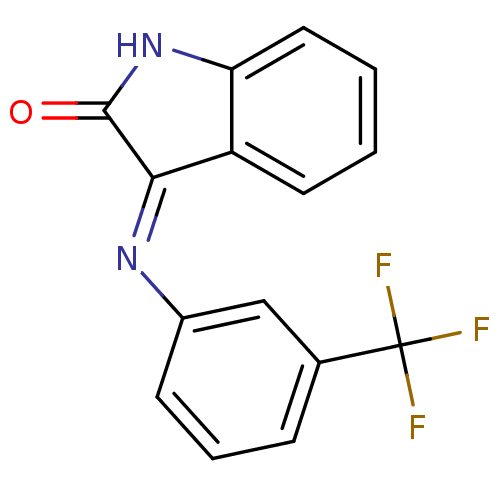 (3-{[3-(trifluoromethyl)phenyl]azamethylene}benzo[d...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 596 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]galanin from human GAL3 | J Med Chem 49: 3757-8 (2006) Article DOI: 10.1021/jm060001n BindingDB Entry DOI: 10.7270/Q2T72H2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 3 (Homo sapiens (Human)) | BDBM50189599 ((E)-3-(4-chlorophenylimino)indolin-2-one | CHEMBL2...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]galanin from human GAL3 | J Med Chem 49: 3757-8 (2006) Article DOI: 10.1021/jm060001n BindingDB Entry DOI: 10.7270/Q2T72H2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 3 (Homo sapiens (Human)) | BDBM50189601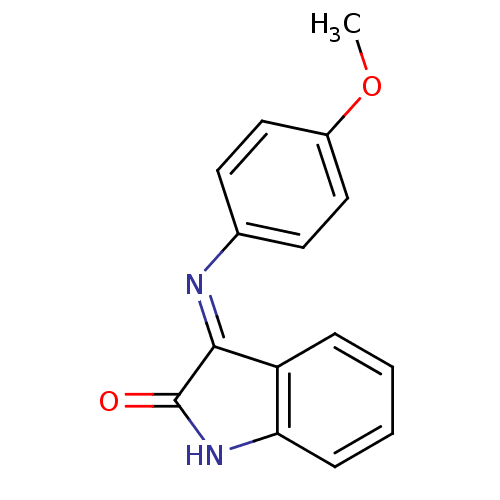 ((E)-3-(4-methoxyphenylimino)indolin-2-one | (E/Z)-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]galanin from human GAL3 | J Med Chem 49: 3757-8 (2006) Article DOI: 10.1021/jm060001n BindingDB Entry DOI: 10.7270/Q2T72H2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50029748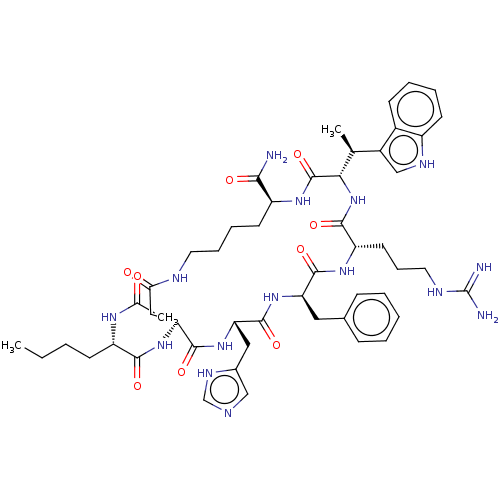 ((2R,3R)-15-(2-Acetylamino-hexanoylamino)-9-benzyl-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description The concentration that inhibits 50% specific binding was determined against the Melanocortin 1 receptor | J Med Chem 38: 4720-9 (1995) BindingDB Entry DOI: 10.7270/Q2B56HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50184359 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description The concentration that inhibits 50% specific binding was determined against the Melanocortin 1 receptor | J Med Chem 38: 4720-9 (1995) BindingDB Entry DOI: 10.7270/Q2B56HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21146 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor was determined in guinea pig cortex using [3H]SNF8702 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50280901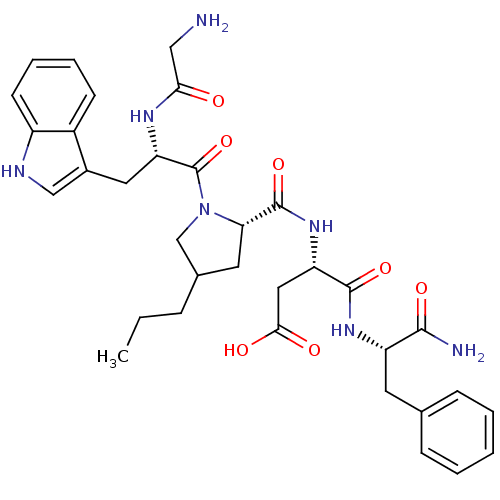 ((S)-3-({1-[(S)-2-(2-Amino-acetylamino)-3-(S)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50454019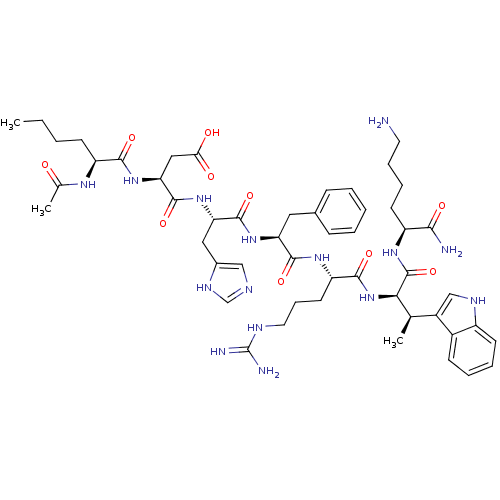 (CHEMBL2370906) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description The concentration that inhibits 50% specific binding was determined against the Melanocortin 1 receptor | J Med Chem 38: 4720-9 (1995) BindingDB Entry DOI: 10.7270/Q2B56HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50092394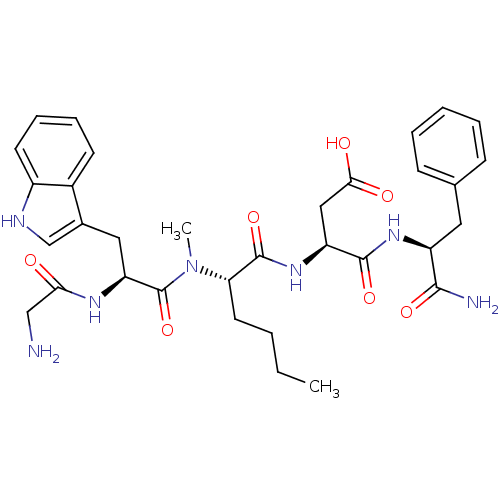 ((S)-3-((S)-2-{[(S)-2-(2-Amino-acetylamino)-3-(S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21146 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50280900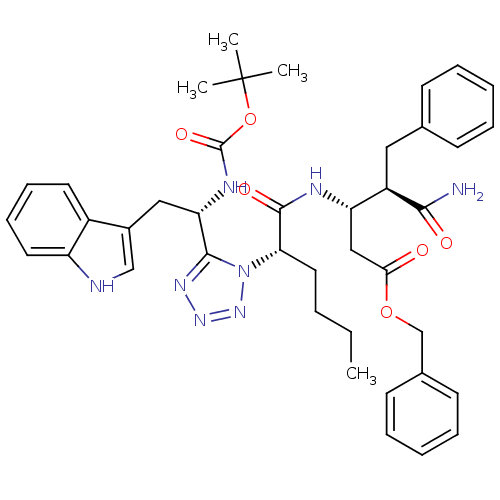 (3-((S)-2-{5-[(S)-1-tert-Butoxycarbonylamino-2-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type A receptor in pancreatic membranes | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50422178 (CHEMBL2112688) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50422175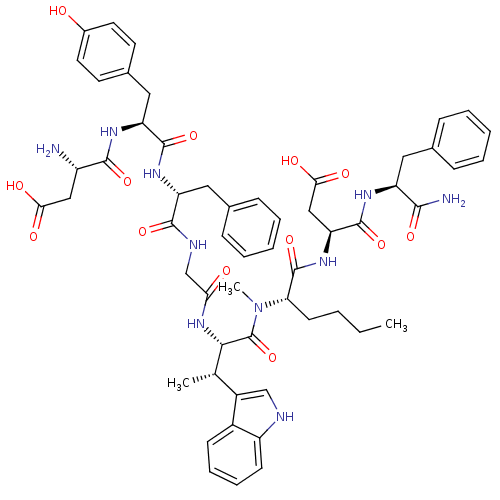 (CHEMBL2112689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor was determined in guinea pig cortex using [3H]SNF8702 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50422175 (CHEMBL2112689) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50280904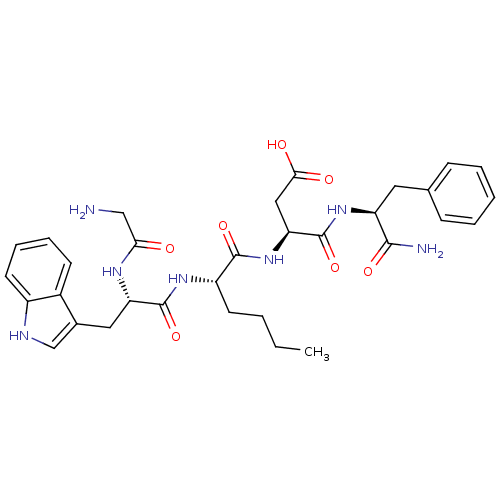 ((S)-3-{(S)-2-[(S)-2-(2-Amino-acetylamino)-3-(S)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50280903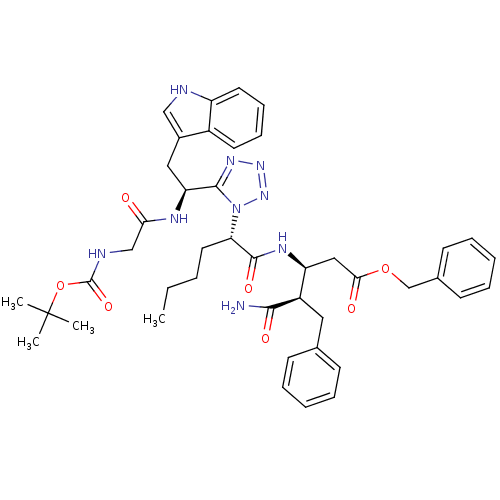 (3-((S)-2-{5-[(S)-1-(2-tert-Butoxycarbonylamino-ace...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 364 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type A receptor in pancreatic membranes | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21146 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 was determined in guinea pig wholebrain using [3H]CTOP radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50422177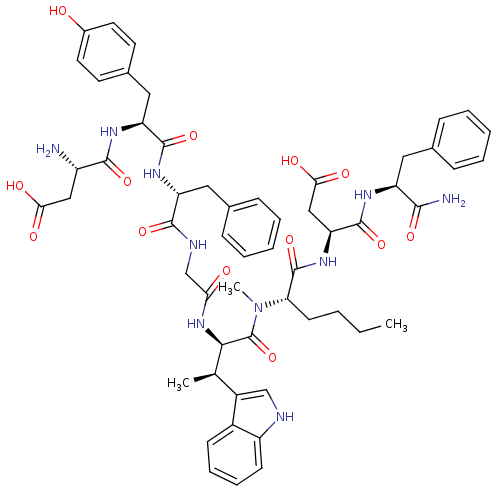 (CHEMBL2111881) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50422178 (CHEMBL2112688) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor was determined in guinea pig pancreatic membranes using [125I]-BH-CCK-8 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50422176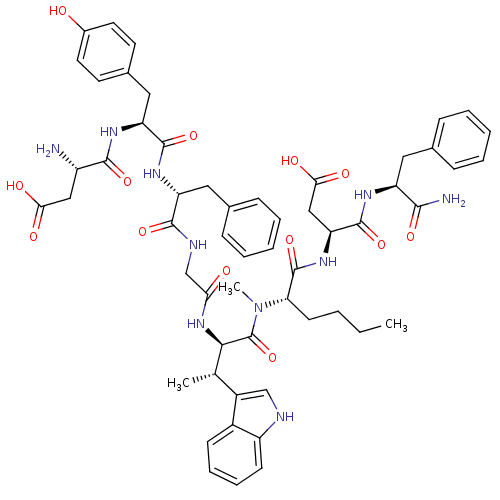 (CHEMBL2111882) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50422175 (CHEMBL2112689) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor was determined in guinea pig pancreatic membranes using [125I]-BH-CCK-8 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50280900 (3-((S)-2-{5-[(S)-1-tert-Butoxycarbonylamino-2-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50422178 (CHEMBL2112688) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor was determined in guinea pig cortex using [3H]SNF8702 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50280904 ((S)-3-{(S)-2-[(S)-2-(2-Amino-acetylamino)-3-(S)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type A receptor in pancreatic membranes | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50280901 ((S)-3-({1-[(S)-2-(2-Amino-acetylamino)-3-(S)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type A receptor in pancreatic membranes | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50422178 (CHEMBL2112688) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 was determined in guinea pig wholebrain using [3H]CTOP radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50280903 (3-((S)-2-{5-[(S)-1-(2-tert-Butoxycarbonylamino-ace...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50280898 ((3S,4R)-3-((S)-2-{5-[(S)-1-(2-Amino-acetylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50422177 (CHEMBL2111881) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor was determined in guinea pig cortex using [3H]SNF8702 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50422176 (CHEMBL2111882) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor was determined in guinea pig cortex using [3H]SNF8702 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50422175 (CHEMBL2112689) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 was determined in guinea pig wholebrain using [3H]CTOP radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 was determined in guinea pig wholebrain using [3H]CTOP radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50422177 (CHEMBL2111881) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor was determined in guinea pig pancreatic membranes using [125I]-BH-CCK-8 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50422176 (CHEMBL2111882) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor was determined in guinea pig pancreatic membranes using [125I]-BH-CCK-8 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50280899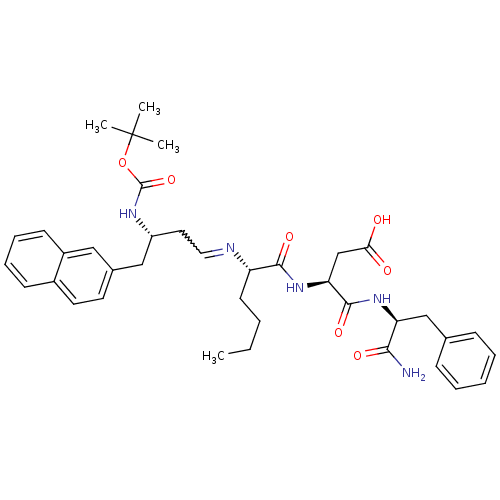 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50280902 (3-{2-Benzyloxycarbonylamino-2-[1-(1-benzyloxycarbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50092394 ((S)-3-((S)-2-{[(S)-2-(2-Amino-acetylamino)-3-(S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type A receptor in pancreatic membranes | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50280902 (3-{2-Benzyloxycarbonylamino-2-[1-(1-benzyloxycarbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type A receptor in pancreatic membranes | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50280898 ((3S,4R)-3-((S)-2-{5-[(S)-1-(2-Amino-acetylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Cholecystokinin type A receptor in pancreatic membranes | Bioorg Med Chem Lett 3: 2011-2016 (1993) Article DOI: 10.1016/S0960-894X(01)81005-2 BindingDB Entry DOI: 10.7270/Q2B27V63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM21146 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor was determined in guinea pig pancreatic membranes using [125I]-BH-CCK-8 as radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50422177 (CHEMBL2111881) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 was determined in guinea pig wholebrain using [3H]CTOP radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50422176 (CHEMBL2111882) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 was determined in guinea pig wholebrain using [3H]CTOP radioligand | J Med Chem 39: 4120-4 (1996) Article DOI: 10.1021/jm960078j BindingDB Entry DOI: 10.7270/Q2P55P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50184359 ((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration against Melanocortin 1 receptor transfected into L-cells was determined by concentration of peptide for 50% maximal cAMP gene... | J Med Chem 38: 4720-9 (1995) BindingDB Entry DOI: 10.7270/Q2B56HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |