

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50220046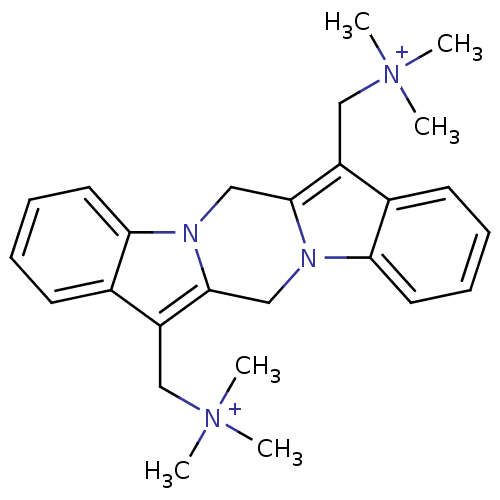 (CHEMBL382243 | trimethyl({20-[(trimethylazaniumyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50220045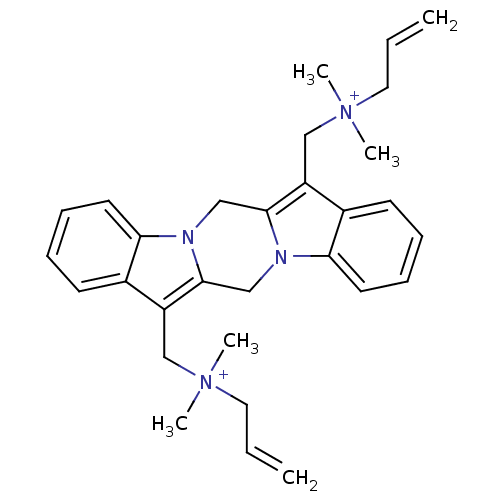 (CHEMBL206666 | [(20-{[dimethyl(prop-2-en-1-yl)azan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50475690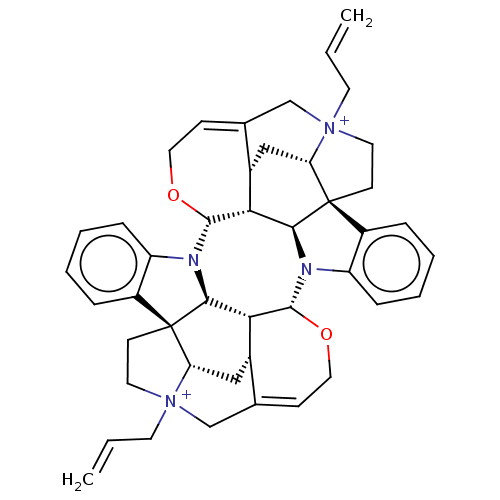 (4,4''-Diallylcaracurinium V Dibromide | Diallylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50475689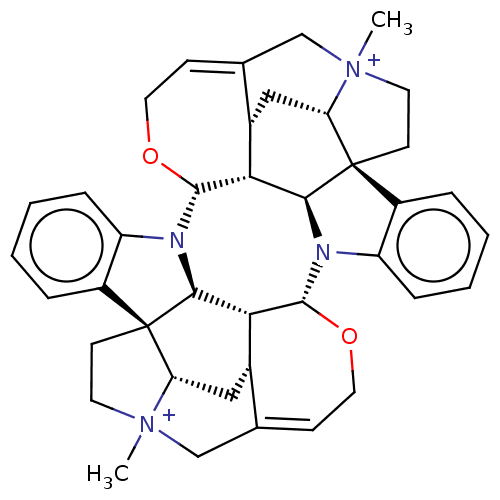 (4,4''-Dimethylcaracurinium V Dichloride | Dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50220060 ((14-dimethylaminomethyl-6H,13H-pyrazino[1,2-a;4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50025037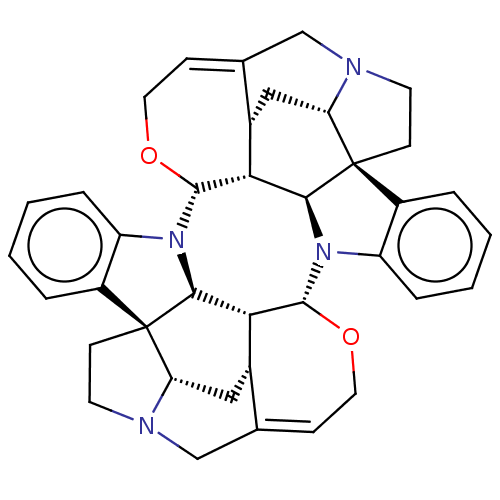 (CHEBI:3382 | Caracurine V) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | <1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50041517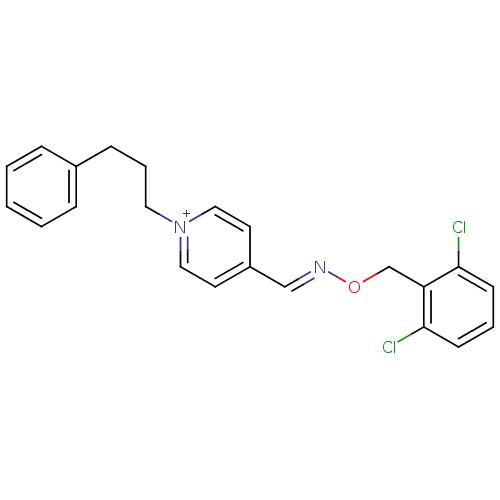 (4-[(2,6-dichloro-benzyloxyimino)-methyl]-1-(3-phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307921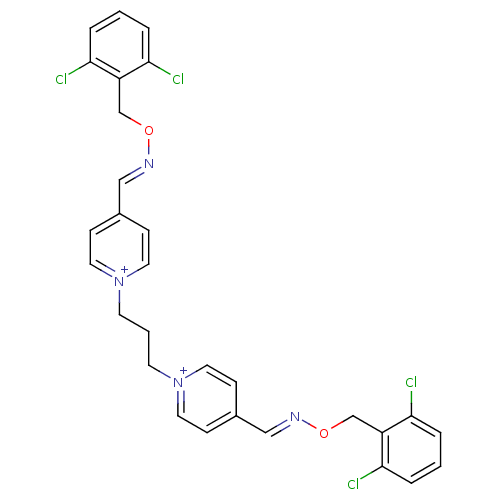 ((E,E)-1,3-bis[4-[[(2,6-dichlorobenzyloxyl)imino]me...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307921 ((E,E)-1,3-bis[4-[[(2,6-dichlorobenzyloxyl)imino]me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50041517 (4-[(2,6-dichloro-benzyloxyimino)-methyl]-1-(3-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307927 (4-((2,6-dichlorobenzylidene)hydrazono)-1-(3-phenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307928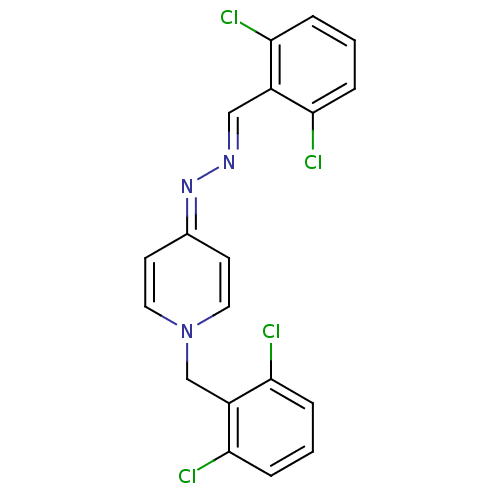 (1-(2,6-dichlorobenzyl)-4-((2,6-dichlorobenzylidene...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307925 (1-benzyl-4-((2,6-dichlorobenzylidene)hydrazono)-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307926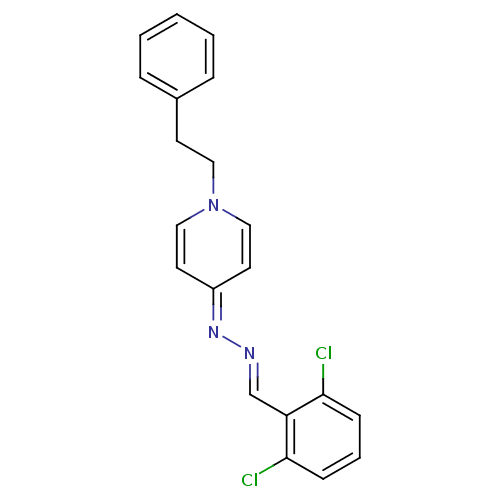 (4-((2,6-dichlorobenzylidene)hydrazono)-1-phenethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307927 (4-((2,6-dichlorobenzylidene)hydrazono)-1-(3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307924 (4-((2,6-dichlorobenzylidene)hydrazono)-1-methyl-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231232 (1-(2-phenylpropyl)-4-oxopiperidine O-(2,6-Dichloro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307925 (1-benzyl-4-((2,6-dichlorobenzylidene)hydrazono)-1,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231234 (1-(3-phenylpropyl)piperidine-4-carbaldehyde O-2,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307926 (4-((2,6-dichlorobenzylidene)hydrazono)-1-phenethyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50231232 (1-(2-phenylpropyl)-4-oxopiperidine O-(2,6-Dichloro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307923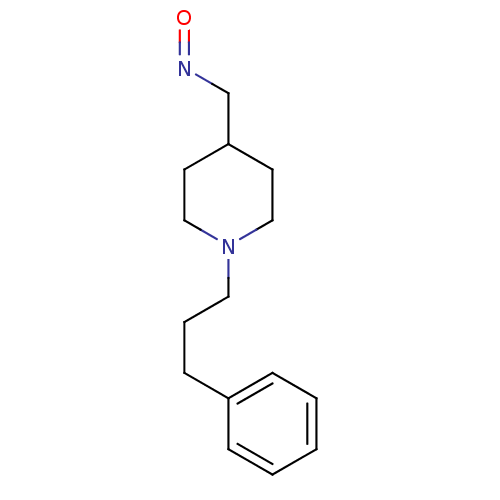 (1-(3-phenylpropyl)piperidine-4-carbaldehyde oxime ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307924 (4-((2,6-dichlorobenzylidene)hydrazono)-1-methyl-1,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50220046 (CHEMBL382243 | trimethyl({20-[(trimethylazaniumyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 229 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in Na,K,Pi buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50475690 (4,4''-Diallylcaracurinium V Dibromide | Diallylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in Na,K,Pi buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50220045 (CHEMBL206666 | [(20-{[dimethyl(prop-2-en-1-yl)azan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in Na,K,Pi buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50220046 (CHEMBL382243 | trimethyl({20-[(trimethylazaniumyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50025037 (CHEBI:3382 | Caracurine V) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 437 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in Na,K,Pi buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50475689 (4,4''-Dimethylcaracurinium V Dichloride | Dimethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in Na,K,Pi buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50220060 ((14-dimethylaminomethyl-6H,13H-pyrazino[1,2-a;4,5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 447 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in Na,K,Pi buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50220060 ((14-dimethylaminomethyl-6H,13H-pyrazino[1,2-a;4,5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50220045 (CHEMBL206666 | [(20-{[dimethyl(prop-2-en-1-yl)azan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||