

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by Lineweaver-Burk plot | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50255702 (CHEMBL520612 | N-(4'-Methyl phenyl)-6,8-dimethoxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by Lineweaver-Burk plot | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50255812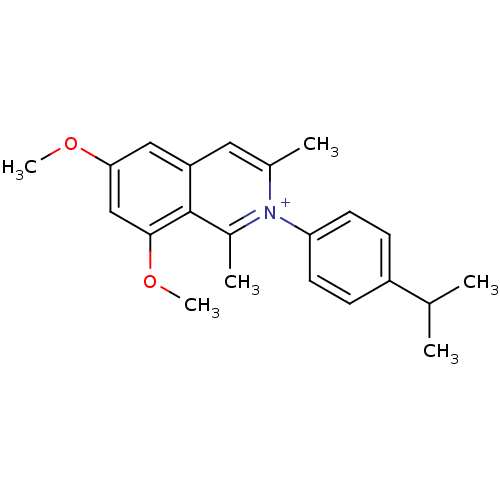 (CHEMBL481022 | N-(4'-i-Propylphenyl)-6,8-dimethoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by Lineweaver-Burk plot | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50198075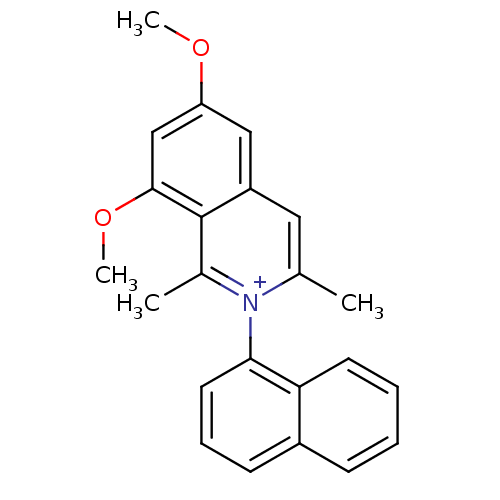 (6,8-dimethoxy-1,3-dimethyl-2-(naphthalen-1-yl)isoq...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by Lineweaver-Burk plot | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50160078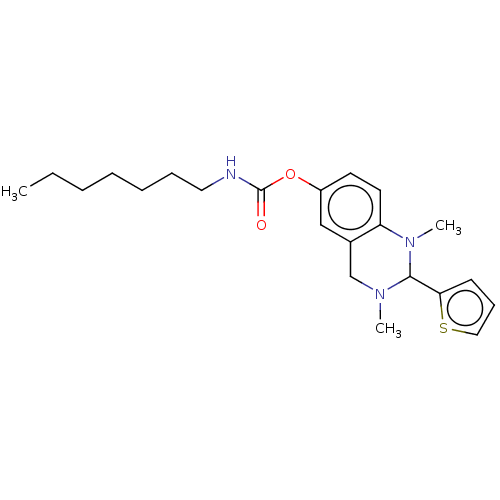 (CHEMBL3787613) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured a... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160078 (CHEMBL3787613) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in baculovirus-infected insect cell system | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160250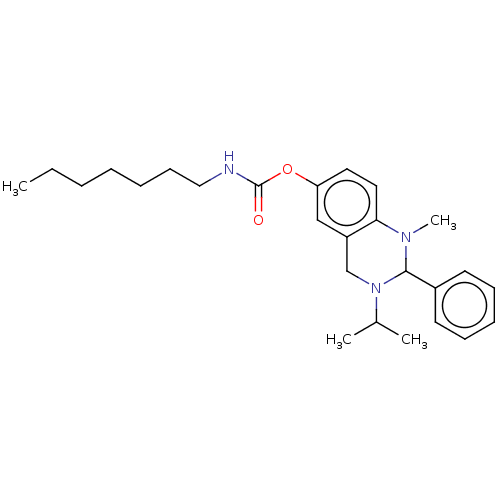 (CHEMBL3785472) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160255 (CHEMBL3785189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160077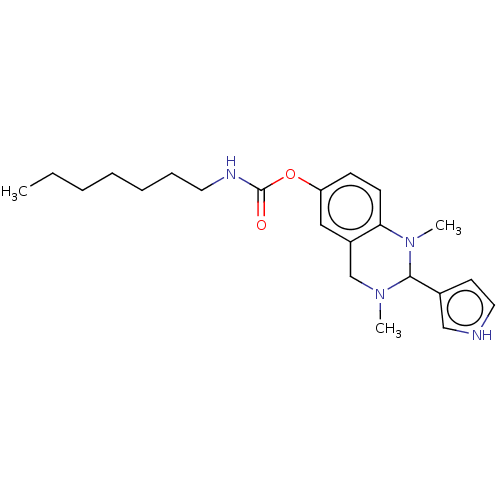 (CHEMBL3785861) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured aft... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160247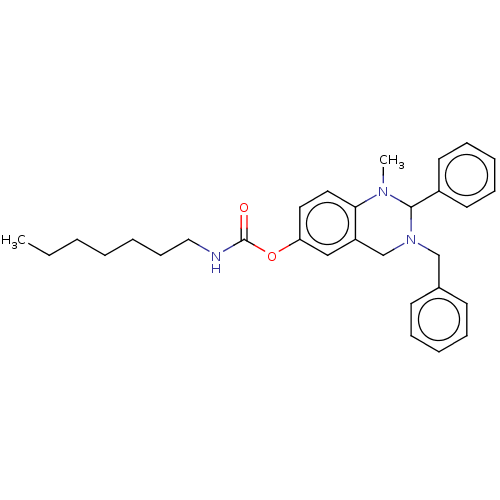 (CHEMBL3786119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160249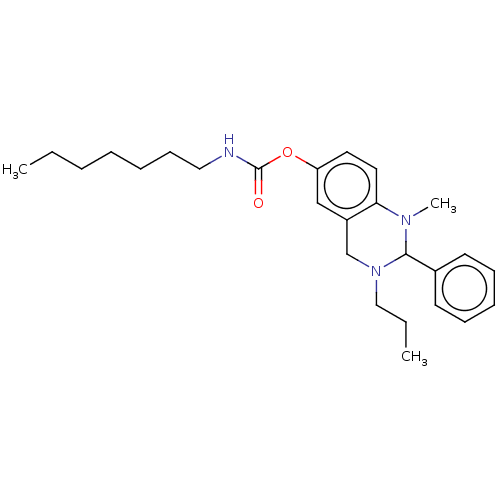 (CHEMBL3785181) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160076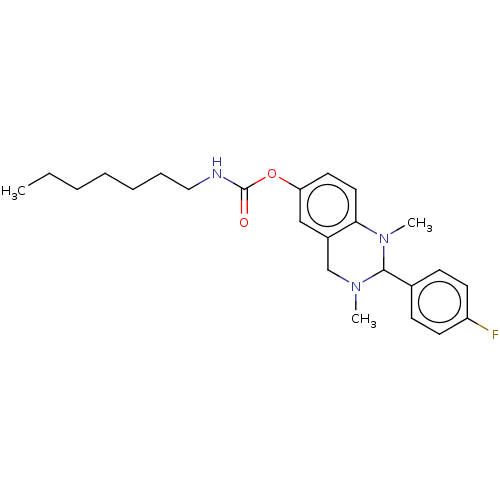 (CHEMBL3787116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50255702 (CHEMBL520612 | N-(4'-Methyl phenyl)-6,8-dimethoxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in baculovirus-infected insect cell system | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160254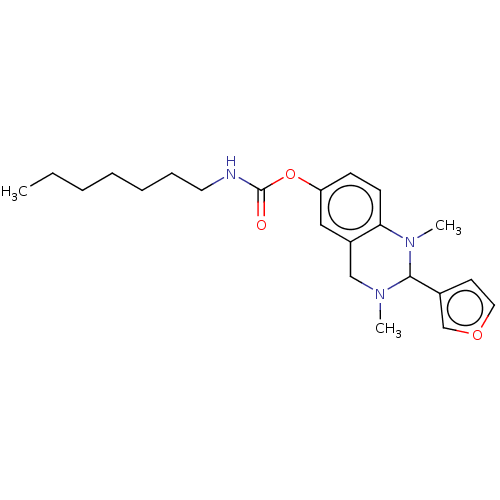 (CHEMBL3786737) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160266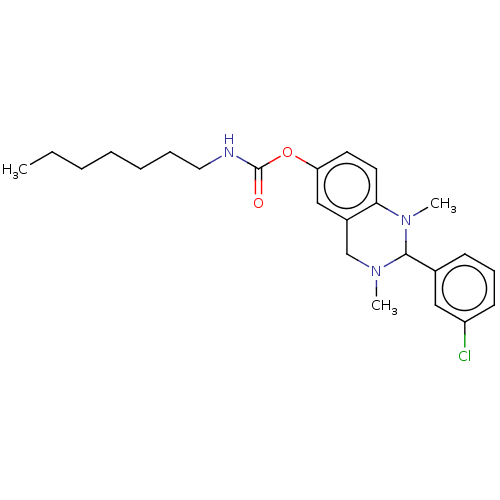 (CHEMBL3787106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160268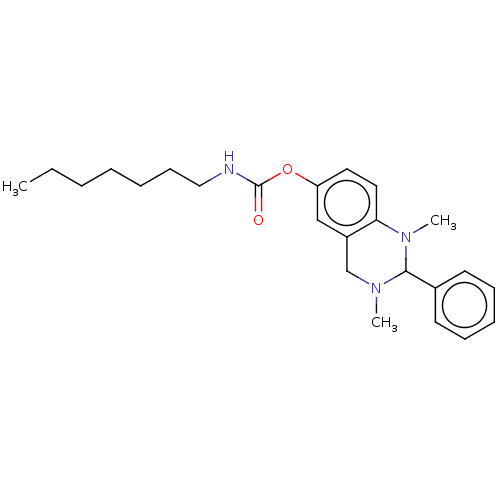 (CHEMBL3786381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50255812 (CHEMBL481022 | N-(4'-i-Propylphenyl)-6,8-dimethoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in baculovirus-infected insect cell system | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160267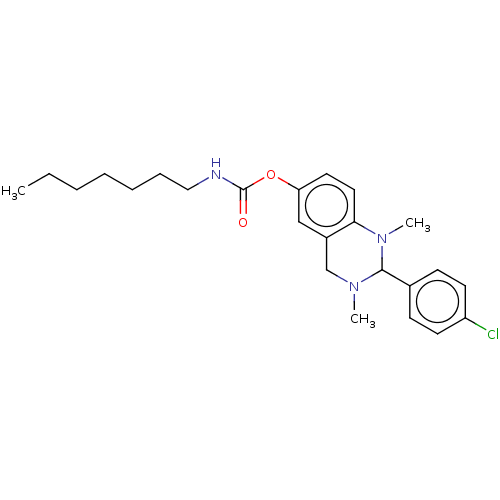 (CHEMBL3785533) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160263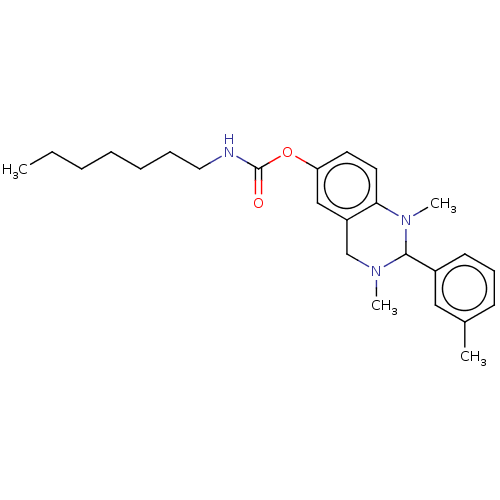 (CHEMBL3786599) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160260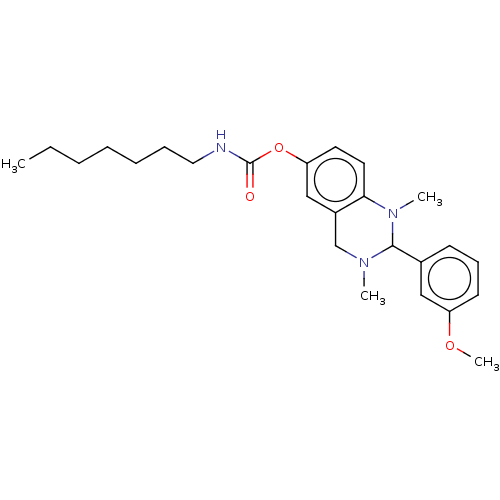 (CHEMBL3786761) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160264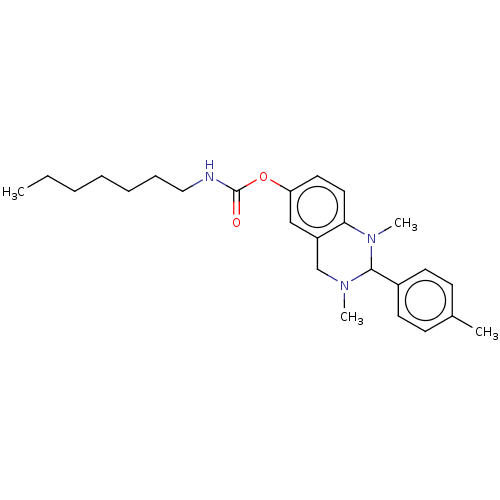 (CHEMBL3787432) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160259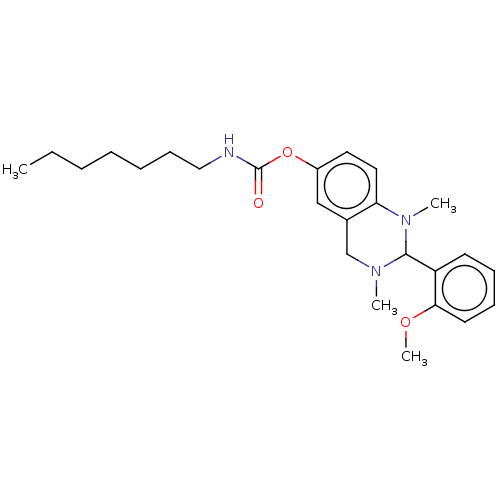 (CHEMBL3785401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160262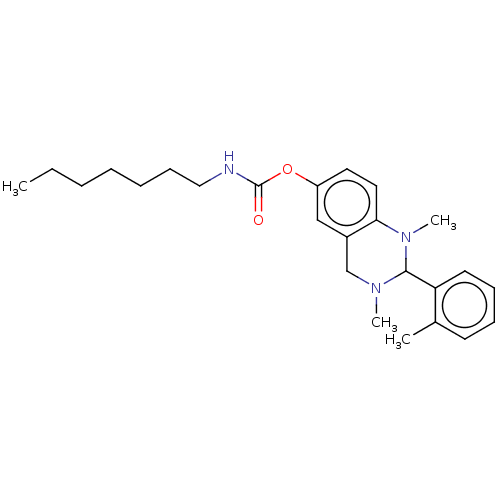 (CHEMBL3787015) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160252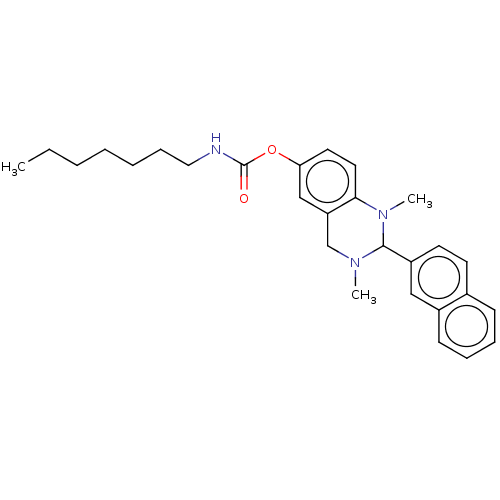 (CHEMBL3786979) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 374 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160265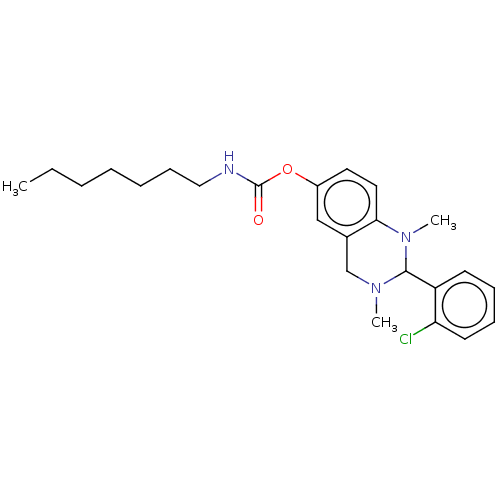 (CHEMBL3785143) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 474 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160251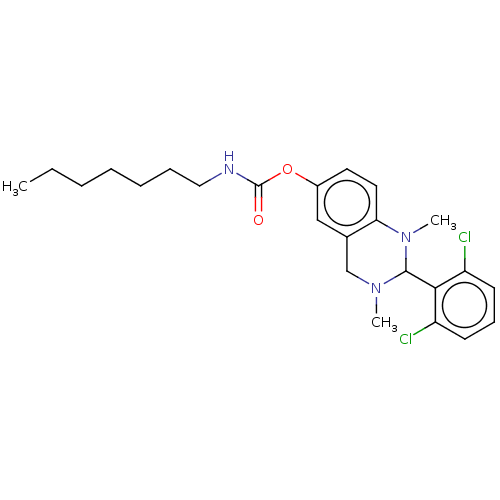 (CHEMBL3785766) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 531 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160256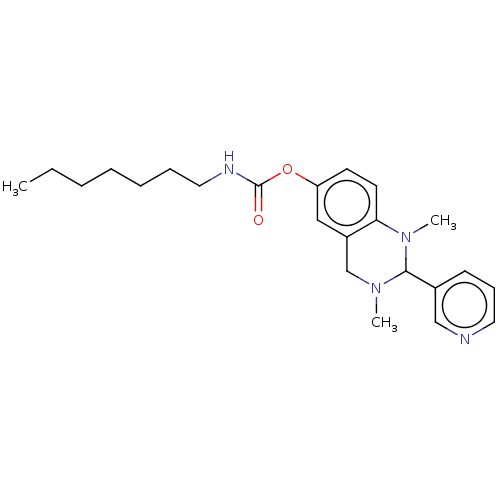 (CHEMBL3785190) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 565 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160257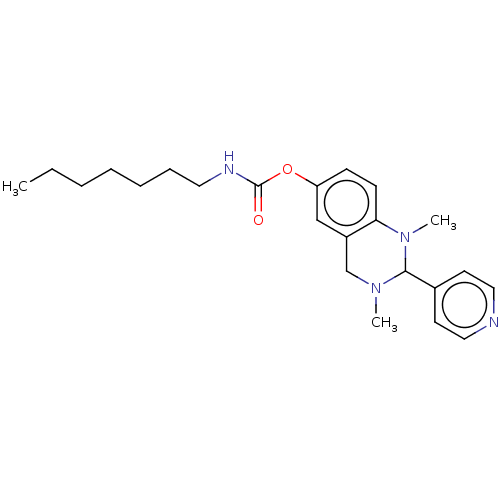 (CHEMBL3787554) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50160077 (CHEMBL3785861) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 852 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured aft... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160261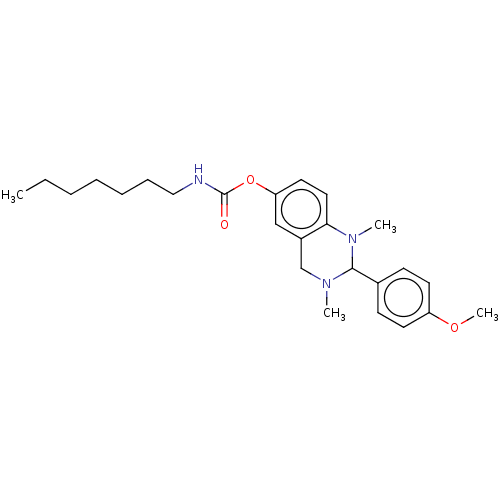 (CHEMBL3785495) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 875 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50198075 (6,8-dimethoxy-1,3-dimethyl-2-(naphthalen-1-yl)isoq...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in baculovirus-infected insect cell system | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50153015 ((-)-Epicatechin-3-gallate | (-)-epicatechin 3-O-ga...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universita£t Curated by ChEMBL | Assay Description Inhibition of p38alpha after 1 hr by ELISA | J Nat Prod 73: 2035-41 (2010) Article DOI: 10.1021/np100523s BindingDB Entry DOI: 10.7270/Q29P31XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50160076 (CHEMBL3787116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured aft... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160245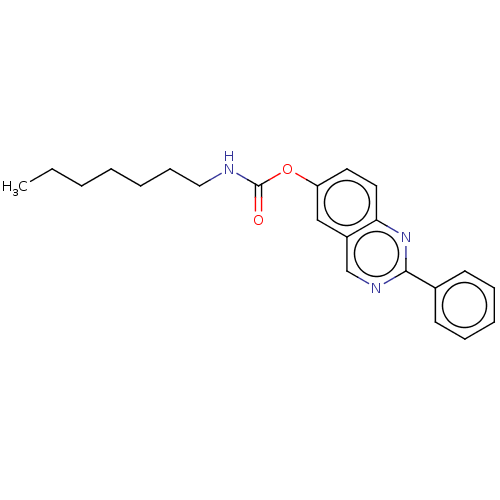 (CHEMBL3785462) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160086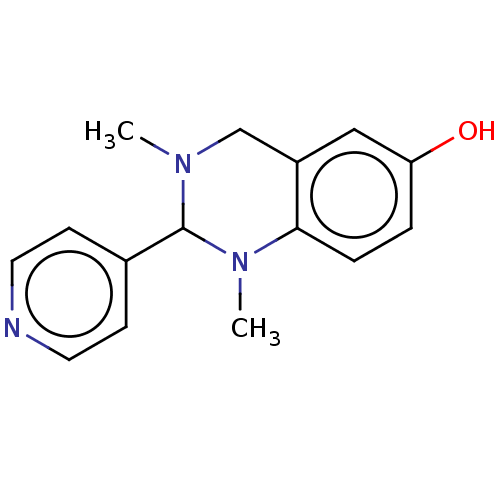 (CHEMBL3786447) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 4.5 mins followed by substrate addition me... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160181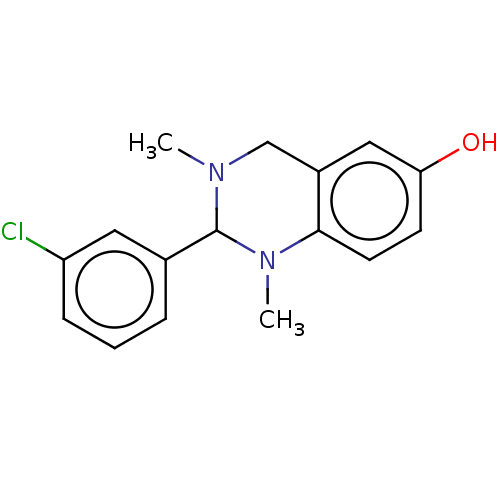 (CHEMBL3786505) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 4.5 mins followed by substrate addition me... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universita£t Curated by ChEMBL | Assay Description Inhibition of p38alpha after 1 hr by ELISA | J Nat Prod 73: 2035-41 (2010) Article DOI: 10.1021/np100523s BindingDB Entry DOI: 10.7270/Q29P31XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160258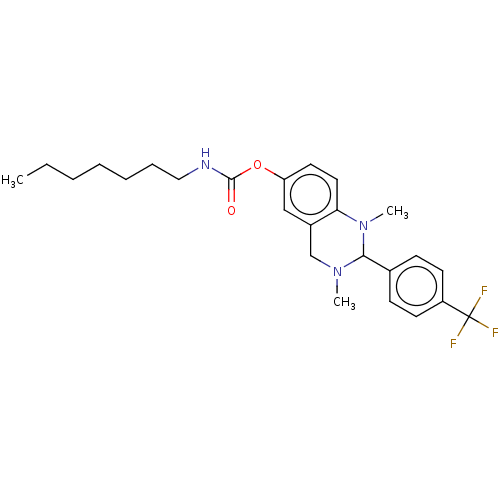 (CHEMBL3787268) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160082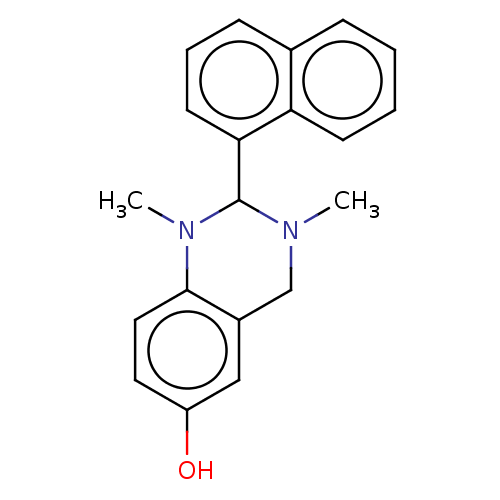 (CHEMBL3785481) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 4.5 mins followed by substrate addition me... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160081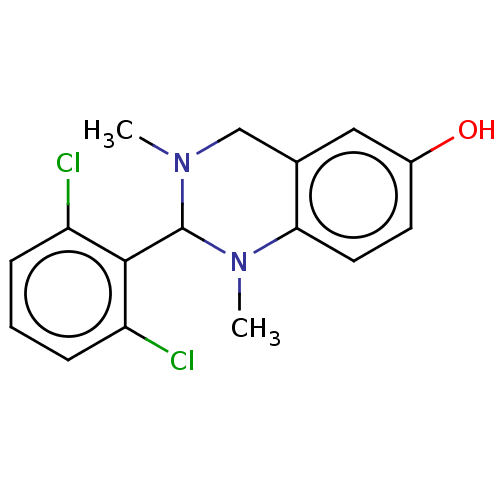 (CHEMBL3786666) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 4.5 mins followed by substrate addition me... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160275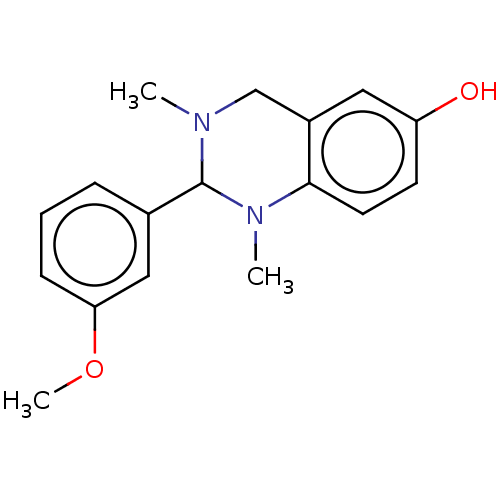 (CHEMBL3785561) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 4.5 mins followed by substrate addition me... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160088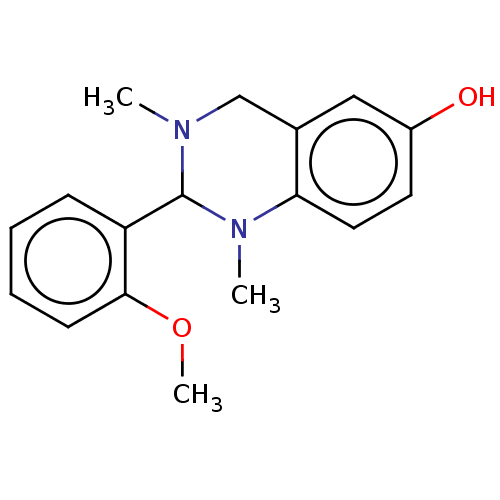 (CHEMBL3785229) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 4.5 mins followed by substrate addition me... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50269144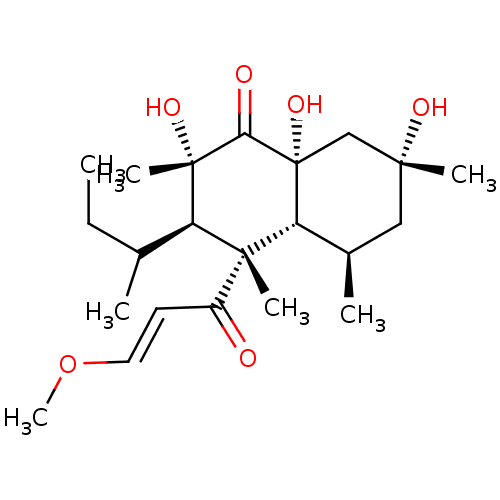 (10-hydroxy-18-methoxybetaenone | CHEMBL498247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR kinase expressed in Sf9 insect cells | J Nat Prod 63: 739-45 (2000) BindingDB Entry DOI: 10.7270/Q2PK0FWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50269144 (10-hydroxy-18-methoxybetaenone | CHEMBL498247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Curated by ChEMBL | Assay Description Inhibition of human recombinant CDK4/cyclin D1 kinase expressed in Sf9 insect cells | J Nat Prod 63: 739-45 (2000) BindingDB Entry DOI: 10.7270/Q2PK0FWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50160081 (CHEMBL3786666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylcholine iodide as substrate preincubated for 4.5 mins followed by substrate addition measured af... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160237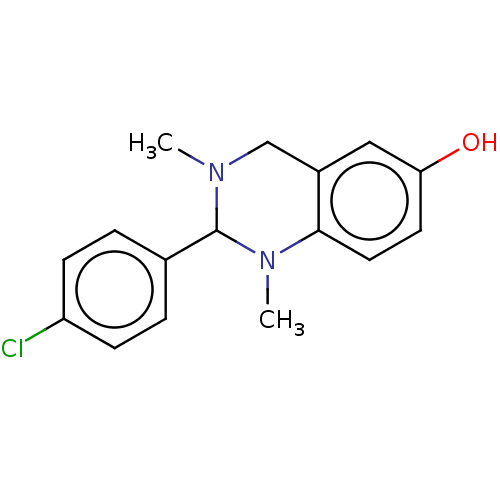 (CHEMBL3785644) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 4.5 mins followed by substrate addition me... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160270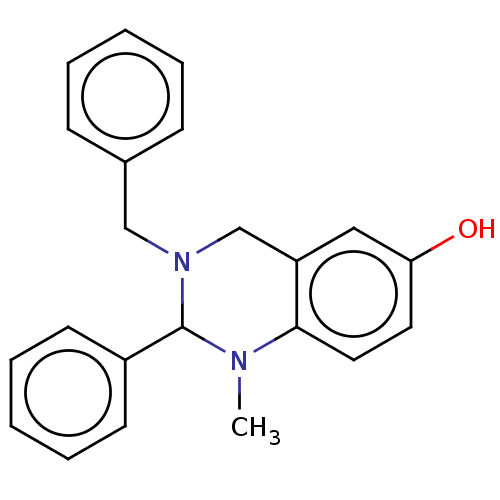 (CHEMBL3786576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 4.5 mins followed by substrate addition me... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 99 total ) | Next | Last >> |