 Found 1620 hits with Last Name = 'brown' and Initial = 't'
Found 1620 hits with Last Name = 'brown' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471498
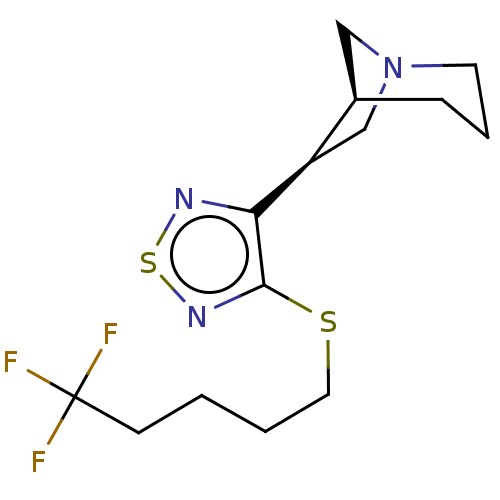
(CHEMBL150450)Show SMILES [H][C@@]12CN(C[C@H]1c1nsnc1SCCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C14H20F3N3S2/c15-14(16,17)5-1-2-7-21-13-12(18-22-19-13)11-9-20-6-3-4-10(11)8-20/h10-11H,1-9H2/t10-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471487
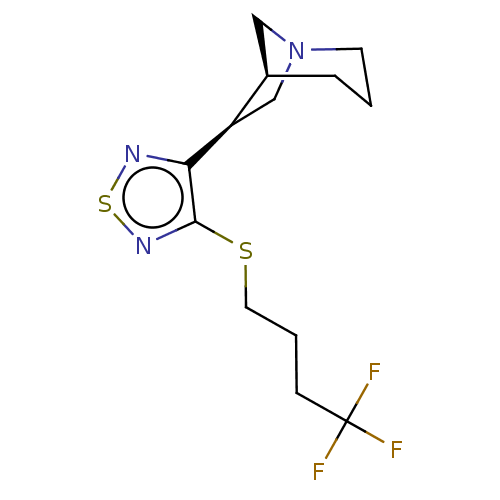
(CHEMBL436075)Show SMILES [H][C@@]12CN(C[C@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471494
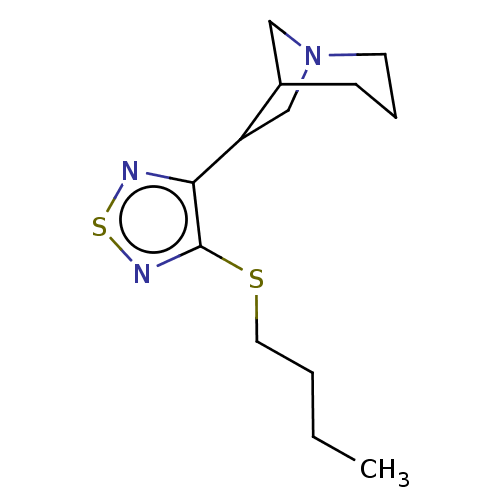
(CHEMBL153723)Show InChI InChI=1S/C13H21N3S2/c1-2-3-7-17-13-12(14-18-15-13)11-9-16-6-4-5-10(11)8-16/h10-11H,2-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471485
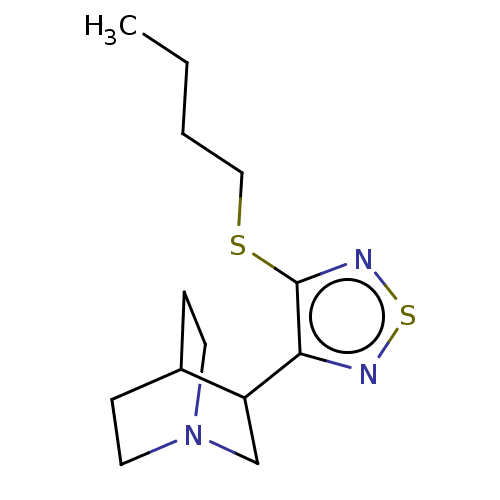
(Vedaclidine)Show SMILES CCCCSc1nsnc1C1CN2CCC1CC2 |(16.46,-2.74,;15.65,-4.05,;14.12,-3.99,;13.3,-5.3,;11.76,-5.24,;10.94,-6.55,;11.52,-7.98,;10.35,-8.98,;9.04,-8.15,;9.4,-6.67,;8.41,-5.49,;6.9,-5.77,;5.93,-4.58,;6.45,-3.12,;7.96,-2.86,;8.95,-4.05,;7.8,-3.47,;7.42,-4.98,)| Show InChI InChI=1S/C13H21N3S2/c1-2-3-8-17-13-12(14-18-15-13)11-9-16-6-4-10(11)5-7-16/h10-11H,2-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305511
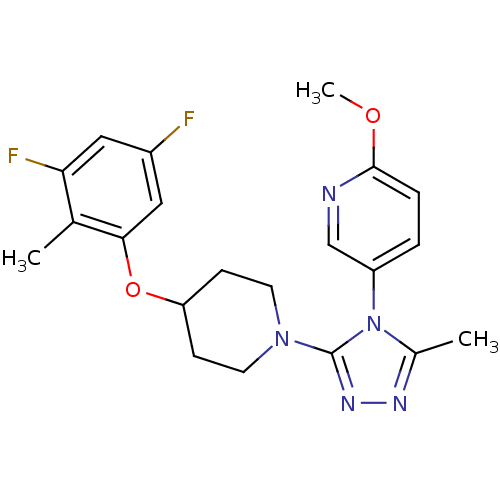
(5-(3-(4-(3,5-difluoro-2-methylphenoxy)piperidin-1-...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1N1CCC(CC1)Oc1cc(F)cc(F)c1C Show InChI InChI=1S/C21H23F2N5O2/c1-13-18(23)10-15(22)11-19(13)30-17-6-8-27(9-7-17)21-26-25-14(2)28(21)16-4-5-20(29-3)24-12-16/h4-5,10-12,17H,6-9H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471499
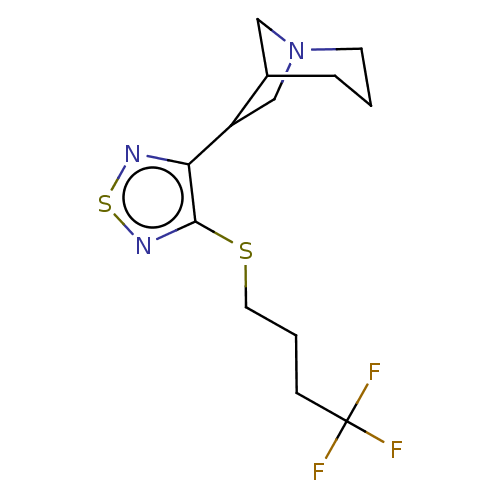
(CHEMBL345774)Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471492
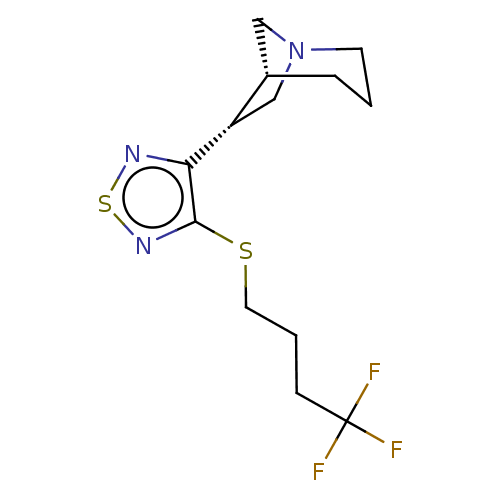
(CHEMBL150293)Show SMILES [H][C@]12CN(C[C@@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471495
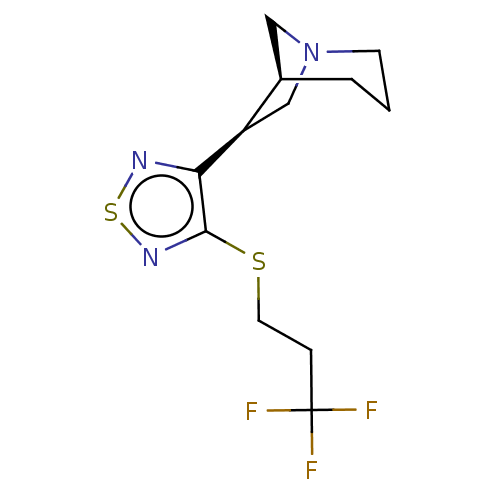
(CHEMBL153478)Show SMILES [H][C@@]12CN(C[C@H]1c1nsnc1SCCC(F)(F)F)CCC2 Show InChI InChI=1S/C12H16F3N3S2/c13-12(14,15)3-5-19-11-10(16-20-17-11)9-7-18-4-1-2-8(9)6-18/h8-9H,1-7H2/t8-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471486
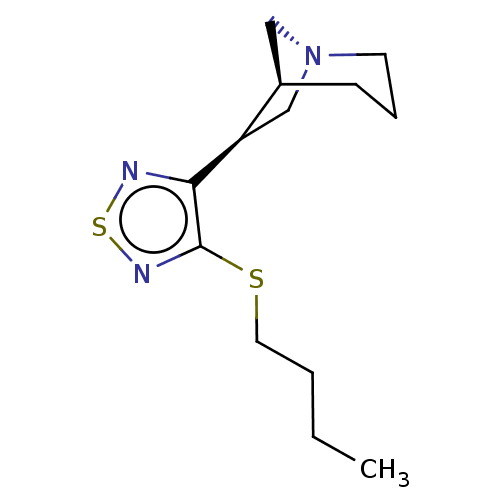
(CHEMBL343731)Show SMILES [H][C@@]12C[N@@](C[C@@]1([H])c1nsnc1SCCCC)CCC2 Show InChI InChI=1S/C13H21N3S2/c1-2-3-7-17-13-12(14-18-15-13)11-9-16-6-4-5-10(11)8-16/h10-11H,2-9H2,1H3/t10-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50033155
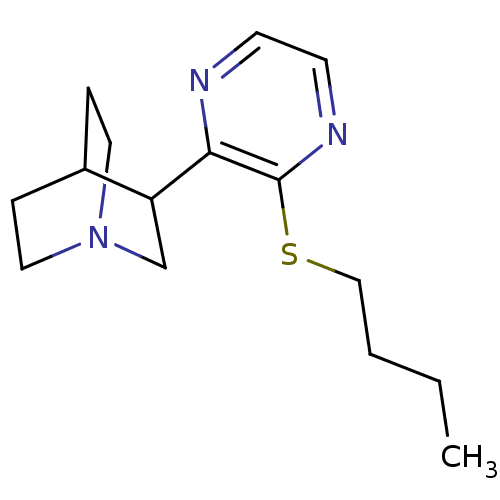
(3-(3-Butylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...)Show SMILES CCCCSc1nccnc1C1CN2CCC1CC2 |(4.41,-8.01,;5.75,-8.78,;5.74,-10.32,;7.07,-11.09,;7.07,-12.63,;8.4,-13.4,;9.74,-12.65,;11.07,-13.42,;11.07,-14.96,;9.73,-15.71,;8.41,-14.94,;7.07,-15.71,;7.07,-17.25,;5.74,-18.01,;6.28,-16.47,;5,-16.36,;5.74,-14.93,;4.42,-15.71,;4.42,-17.25,)| Show InChI InChI=1S/C15H23N3S/c1-2-3-10-19-15-14(16-6-7-17-15)13-11-18-8-4-12(13)5-9-18/h6-7,12-13H,2-5,8-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471491
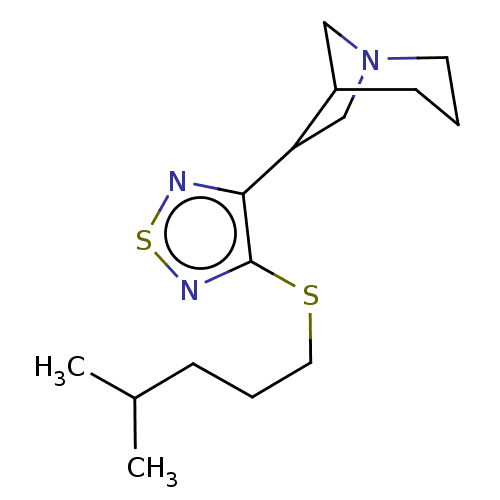
(CHEMBL357279)Show InChI InChI=1S/C15H25N3S2/c1-11(2)5-4-8-19-15-14(16-20-17-15)13-10-18-7-3-6-12(13)9-18/h11-13H,3-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070725
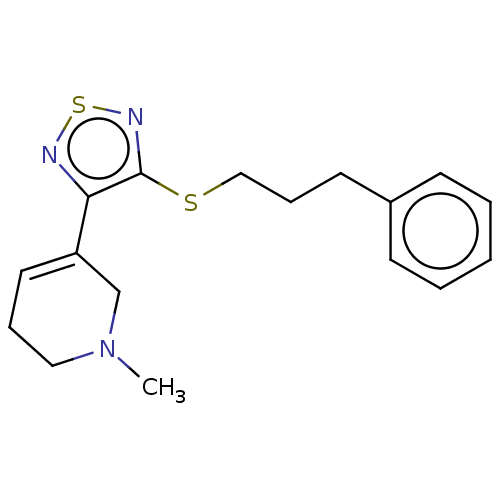
(CHEMBL318403)Show InChI InChI=1S/C28H43N5O8/c1-4-16(3)23(26(38)30-19(5-2)28(40)41)32-25(37)21-10-8-14-33(21)27(39)20(9-6-7-13-29)31-24(36)18-15-17(34)11-12-22(18)35/h11-12,15-16,19-21,23,34-35H,4-10,13-14,29H2,1-3H3,(H,30,38)(H,31,36)(H,32,37)(H,40,41)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003351
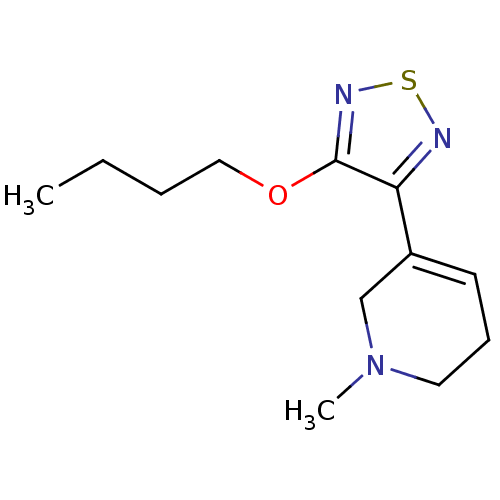
(3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...)Show InChI InChI=1S/C12H19N3OS/c1-3-4-8-16-12-11(13-17-14-12)10-6-5-7-15(2)9-10/h6H,3-5,7-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305512

(2-methoxy-5-(3-(methoxymethyl)-5-(4-(o-tolyloxy)pi...)Show SMILES COCc1nnc(N2CCC(CC2)Oc2ccccc2C)n1-c1ccc(OC)nc1 Show InChI InChI=1S/C22H27N5O3/c1-16-6-4-5-7-19(16)30-18-10-12-26(13-11-18)22-25-24-20(15-28-2)27(22)17-8-9-21(29-3)23-14-17/h4-9,14,18H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305510

(5-(3-(4-(3-fluoro-2-methylphenoxy)piperidin-1-yl)-...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1N1CCC(CC1)Oc1cccc(F)c1C Show InChI InChI=1S/C21H24FN5O2/c1-14-18(22)5-4-6-19(14)29-17-9-11-26(12-10-17)21-25-24-15(2)27(21)16-7-8-20(28-3)23-13-16/h4-8,13,17H,9-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471489
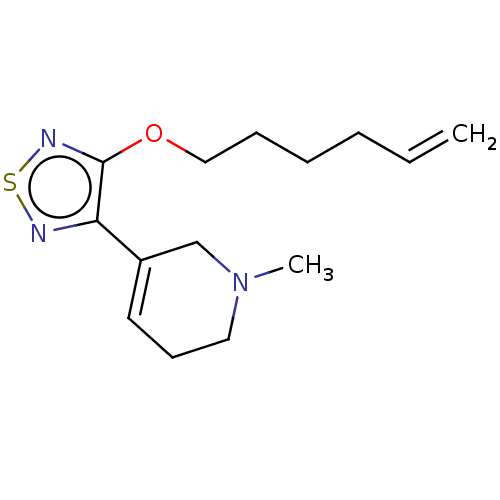
(CHEMBL153150)Show InChI InChI=1S/C14H21N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h3,8H,1,4-7,9-11H2,2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471496
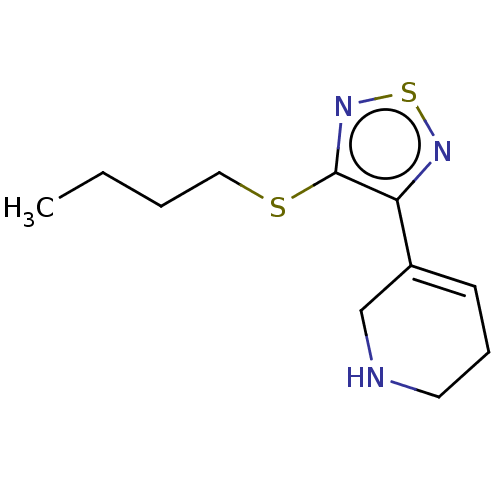
(CHEMBL153031)Show InChI InChI=1S/C11H17N3S2/c1-2-3-7-15-11-10(13-16-14-11)9-5-4-6-12-8-9/h5,12H,2-4,6-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50470855
(CHEMBL60708)Show InChI InChI=1S/C23H24N2O2/c1-27-22-14-19-10-6-5-9-18(19)13-21(22)23(26)24-20-11-12-25(16-20)15-17-7-3-2-4-8-17/h2-10,13-14,20H,11-12,15-16H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand |
J Med Chem 39: 1946-8 (1996)
Article DOI: 10.1021/jm960017l
BindingDB Entry DOI: 10.7270/Q2R49THW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50033151
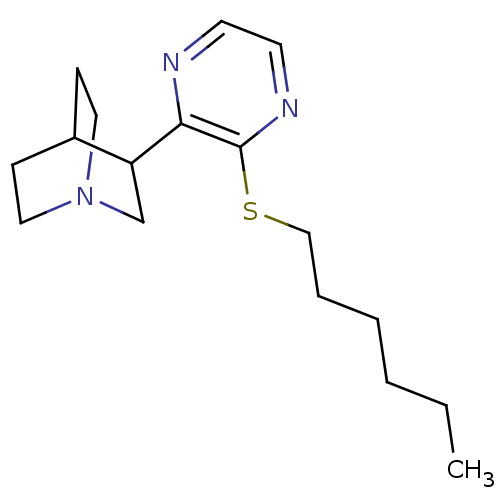
(3-(3-Hexylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...)Show SMILES CCCCCCSc1nccnc1C1CN2CCC1CC2 |(4.09,3.27,;5.44,2.49,;5.44,.95,;6.77,.18,;6.77,-1.36,;8.1,-2.13,;8.1,-3.67,;9.43,-4.44,;10.76,-3.69,;12.09,-4.46,;12.09,-6,;10.76,-6.75,;9.43,-5.98,;8.1,-6.75,;8.1,-8.29,;6.77,-9.05,;7.31,-7.51,;6.03,-7.4,;6.77,-5.97,;5.44,-6.75,;5.44,-8.29,)| Show InChI InChI=1S/C17H27N3S/c1-2-3-4-5-12-21-17-16(18-8-9-19-17)15-13-20-10-6-14(15)7-11-20/h8-9,14-15H,2-7,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305509
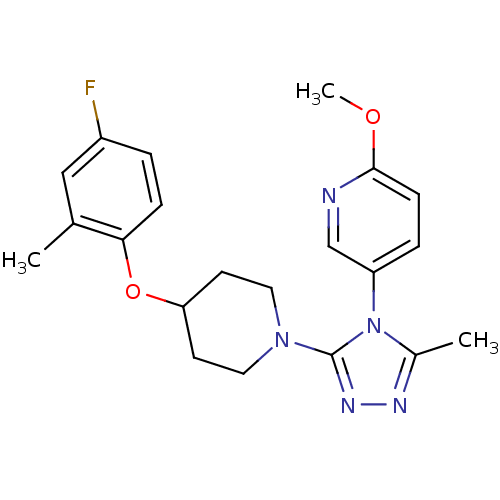
(5-(3-(4-(4-fluoro-2-methylphenoxy)piperidin-1-yl)-...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1N1CCC(CC1)Oc1ccc(F)cc1C Show InChI InChI=1S/C21H24FN5O2/c1-14-12-16(22)4-6-19(14)29-18-8-10-26(11-9-18)21-25-24-15(2)27(21)17-5-7-20(28-3)23-13-17/h4-7,12-13,18H,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315675
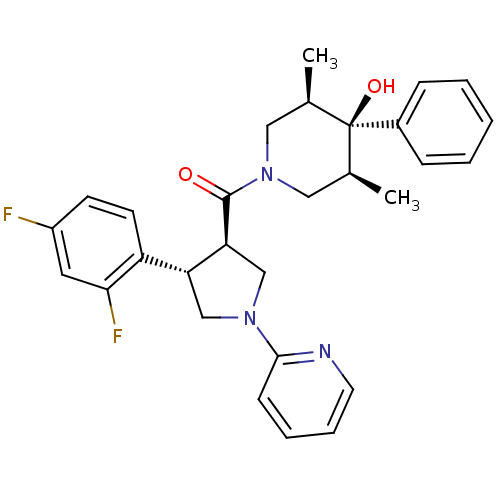
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-2-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1ccccn1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-8-4-3-5-9-21)28(35)25-18-33(27-10-6-7-13-32-27)17-24(25)23-12-11-22(30)14-26(23)31/h3-14,19-20,24-25,36H,15-18H2,1-2H3/t19-,20+,24-,25+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315688
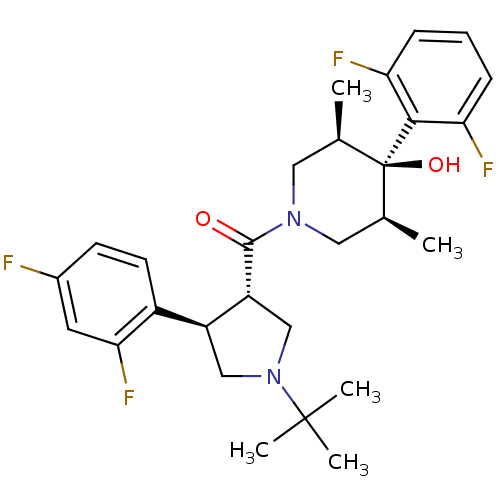
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1c(F)cccc1F)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H34F4N2O2/c1-16-12-33(13-17(2)28(16,36)25-22(30)7-6-8-23(25)31)26(35)21-15-34(27(3,4)5)14-20(21)19-10-9-18(29)11-24(19)32/h6-11,16-17,20-21,36H,12-15H2,1-5H3/t16-,17+,20-,21+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50470850
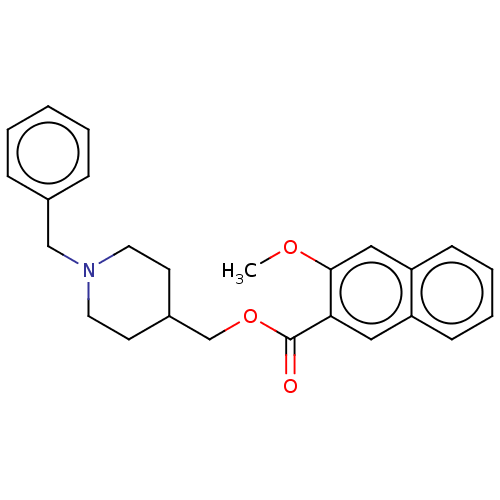
(CHEMBL59326)Show InChI InChI=1S/C25H27NO3/c1-28-24-16-22-10-6-5-9-21(22)15-23(24)25(27)29-18-20-11-13-26(14-12-20)17-19-7-3-2-4-8-19/h2-10,15-16,20H,11-14,17-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand |
J Med Chem 39: 1946-8 (1996)
Article DOI: 10.1021/jm960017l
BindingDB Entry DOI: 10.7270/Q2R49THW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315674
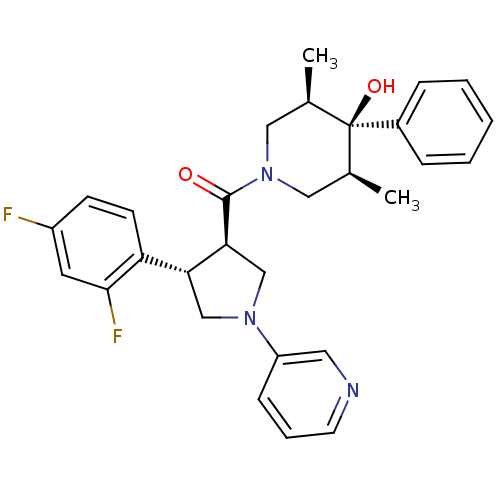
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-3-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnc1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-7-4-3-5-8-21)28(35)26-18-33(23-9-6-12-32-14-23)17-25(26)24-11-10-22(30)13-27(24)31/h3-14,19-20,25-26,36H,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315676
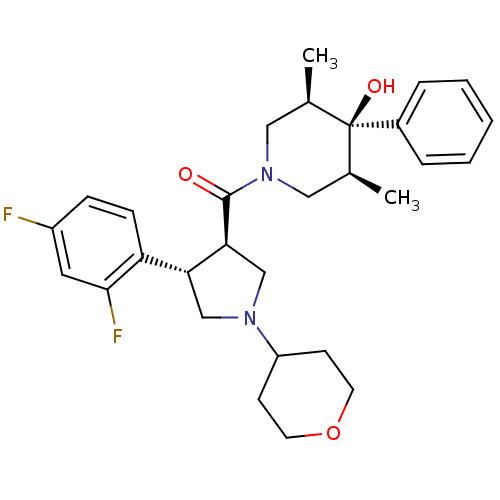
(((3R,4S)-4-(2,4-difluorophenyl)-1-(tetrahydro-2H-p...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCOCC1 |r| Show InChI InChI=1S/C29H36F2N2O3/c1-19-15-33(16-20(2)29(19,35)21-6-4-3-5-7-21)28(34)26-18-32(23-10-12-36-13-11-23)17-25(26)24-9-8-22(30)14-27(24)31/h3-9,14,19-20,23,25-26,35H,10-13,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305508
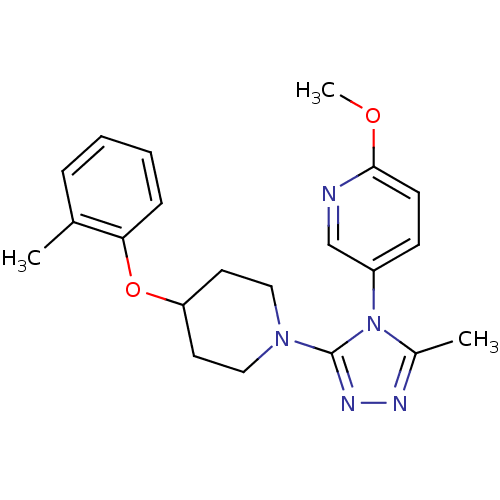
(2-methoxy-5-(3-methyl-5-(4-(o-tolyloxy)piperidin-1...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1N1CCC(CC1)Oc1ccccc1C Show InChI InChI=1S/C21H25N5O2/c1-15-6-4-5-7-19(15)28-18-10-12-25(13-11-18)21-24-23-16(2)26(21)17-8-9-20(27-3)22-14-17/h4-9,14,18H,10-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305518
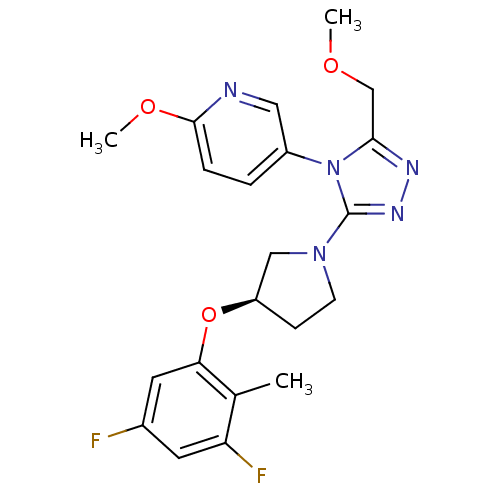
((R)-5-(3-(3-(3,5-difluoro-2-methylphenoxy)pyrrolid...)Show SMILES COCc1nnc(N2CC[C@H](C2)Oc2cc(F)cc(F)c2C)n1-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H23F2N5O3/c1-13-17(23)8-14(22)9-18(13)31-16-6-7-27(11-16)21-26-25-19(12-29-2)28(21)15-4-5-20(30-3)24-10-15/h4-5,8-10,16H,6-7,11-12H2,1-3H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315686
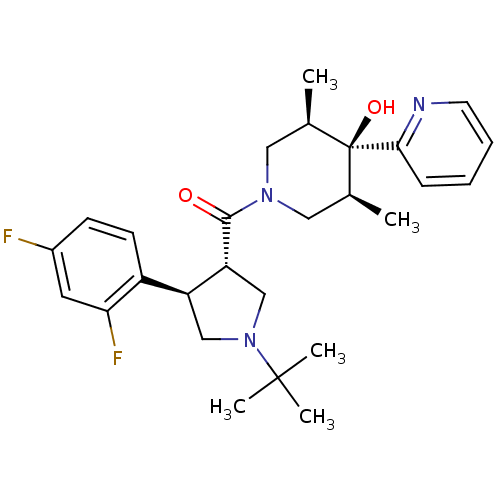
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccn1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C27H35F2N3O2/c1-17-13-31(14-18(2)27(17,34)24-8-6-7-11-30-24)25(33)22-16-32(26(3,4)5)15-21(22)20-10-9-19(28)12-23(20)29/h6-12,17-18,21-22,34H,13-16H2,1-5H3/t17-,18+,21-,22+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315677

(((3R,4S)-1-cyclobutyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCC1 |r| Show InChI InChI=1S/C28H34F2N2O2/c1-18-14-32(15-19(2)28(18,34)20-7-4-3-5-8-20)27(33)25-17-31(22-9-6-10-22)16-24(25)23-12-11-21(29)13-26(23)30/h3-5,7-8,11-13,18-19,22,24-25,34H,6,9-10,14-17H2,1-2H3/t18-,19+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50262270
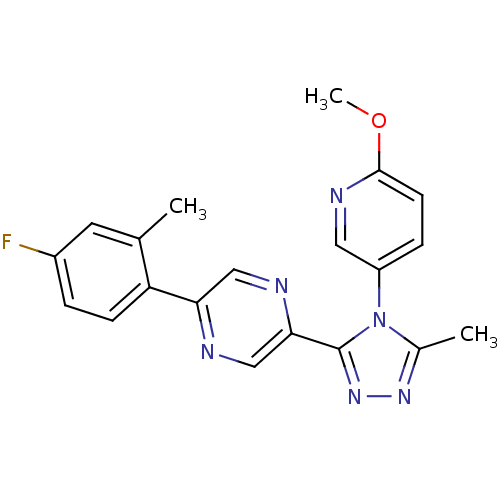
(2-(4-fluoro-2-methylphenyl)-5-(4-(6-methoxypyridin...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(cn1)-c1ccc(F)cc1C Show InChI InChI=1S/C20H17FN6O/c1-12-8-14(21)4-6-16(12)17-10-23-18(11-22-17)20-26-25-13(2)27(20)15-5-7-19(28-3)24-9-15/h4-11H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50263857

((Z)-4-ethoxy-N-(2-oxo-2-(2-(2-oxoindolin-3-ylidene...)Show SMILES CCOc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccccc1 Show InChI InChI=1S/C24H22N4O5S/c1-2-33-18-12-14-19(15-13-18)34(31,32)28(17-8-4-3-5-9-17)16-22(29)26-27-23-20-10-6-7-11-21(20)25-24(23)30/h3-15H,2,16H2,1H3,(H,26,29)(H,25,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human oxytocin receptor by cell based beta-lactamase reporter gene assay |
Bioorg Med Chem Lett 18: 5242-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.066
BindingDB Entry DOI: 10.7270/Q2833RVK |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50470854
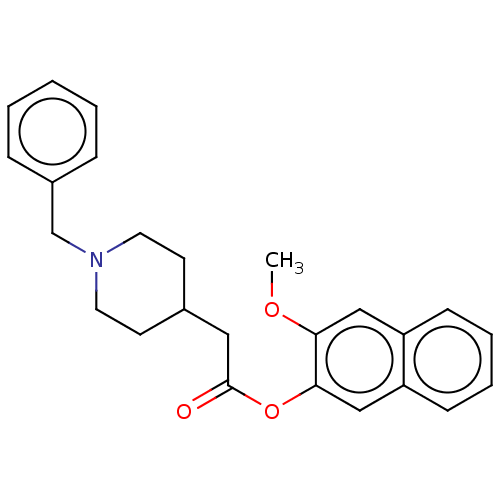
(CHEMBL433255)Show InChI InChI=1S/C25H27NO3/c1-28-23-16-21-9-5-6-10-22(21)17-24(23)29-25(27)15-19-11-13-26(14-12-19)18-20-7-3-2-4-8-20/h2-10,16-17,19H,11-15,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand |
J Med Chem 39: 1946-8 (1996)
Article DOI: 10.1021/jm960017l
BindingDB Entry DOI: 10.7270/Q2R49THW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315680
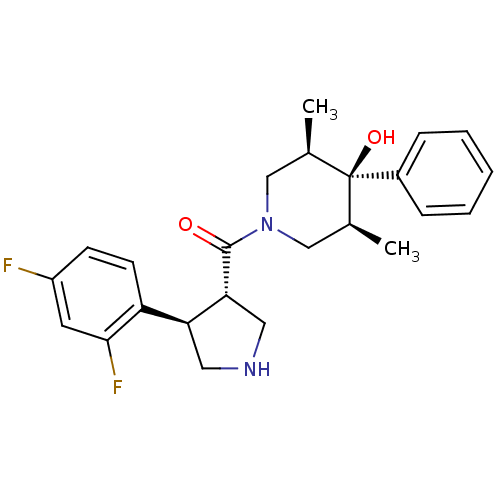
(((3S,4R)-4-(2,4-difluorophenyl)pyrrolidin-3-yl)((3...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CNC[C@H]1c1ccc(F)cc1F |r| Show InChI InChI=1S/C24H28F2N2O2/c1-15-13-28(14-16(2)24(15,30)17-6-4-3-5-7-17)23(29)21-12-27-11-20(21)19-9-8-18(25)10-22(19)26/h3-10,15-16,20-21,27,30H,11-14H2,1-2H3/t15-,16+,20-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315681
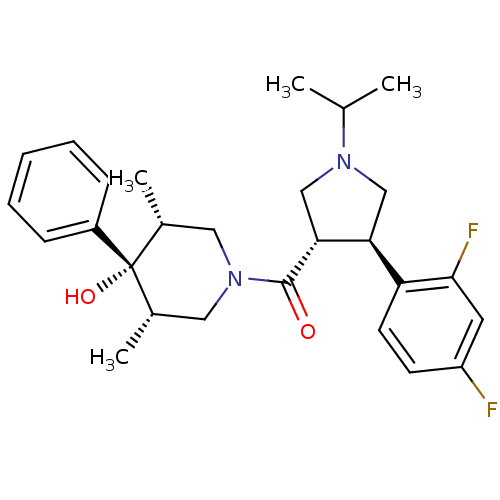
(((3S,4R)-4-(2,4-difluorophenyl)-1-isopropylpyrroli...)Show SMILES CC(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1C[C@H](C)[C@](O)([C@H](C)C1)c1ccccc1 |r| Show InChI InChI=1S/C27H34F2N2O2/c1-17(2)30-15-23(22-11-10-21(28)12-25(22)29)24(16-30)26(32)31-13-18(3)27(33,19(4)14-31)20-8-6-5-7-9-20/h5-12,17-19,23-24,33H,13-16H2,1-4H3/t18-,19+,23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378743
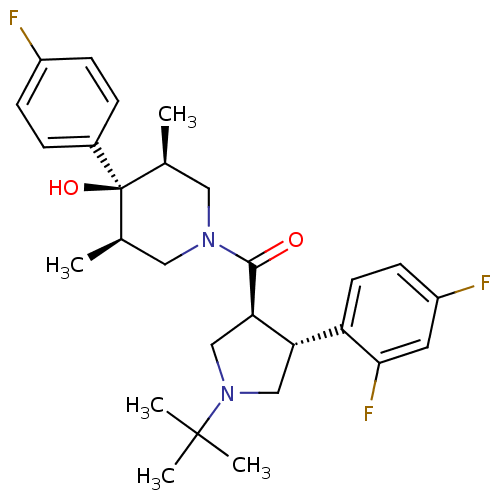
(CHEMBL1204061)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(F)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35F3N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315679

(((3R,4S)-1-cyclopropyl-4-(2,4-difluorophenyl)pyrro...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CC1 |r| Show InChI InChI=1S/C27H32F2N2O2/c1-17-13-31(14-18(2)27(17,33)19-6-4-3-5-7-19)26(32)24-16-30(21-9-10-21)15-23(24)22-11-8-20(28)12-25(22)29/h3-8,11-12,17-18,21,23-24,33H,9-10,13-16H2,1-2H3/t17-,18+,23-,24+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305506
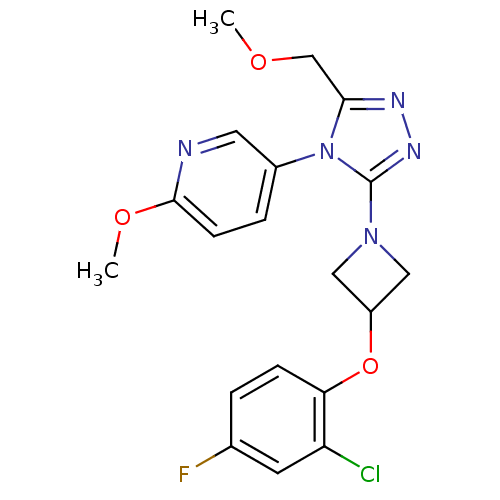
(5-(3-(3-(2-chloro-4-fluorophenoxy)azetidin-1-yl)-5...)Show SMILES COCc1nnc(N2CC(C2)Oc2ccc(F)cc2Cl)n1-c1ccc(OC)nc1 Show InChI InChI=1S/C19H19ClFN5O3/c1-27-11-17-23-24-19(26(17)13-4-6-18(28-2)22-8-13)25-9-14(10-25)29-16-5-3-12(21)7-15(16)20/h3-8,14H,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471497
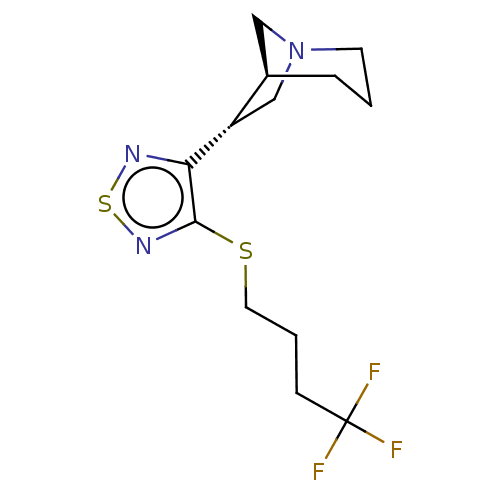
(CHEMBL150501)Show SMILES [H][C@@]12CN(C[C@@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471488
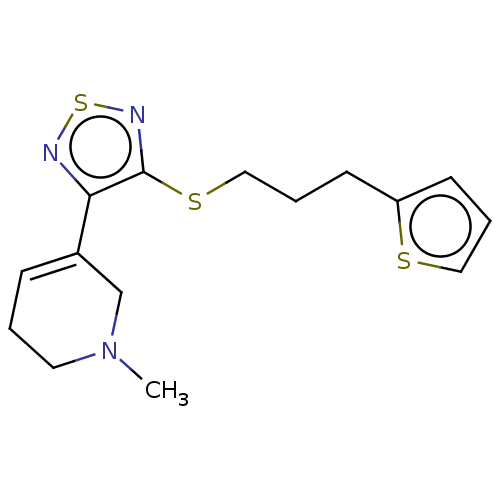
(CHEMBL152892)Show InChI InChI=1S/C15H19N3S3/c1-18-8-2-5-12(11-18)14-15(17-21-16-14)20-10-4-7-13-6-3-9-19-13/h3,5-6,9H,2,4,7-8,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378747
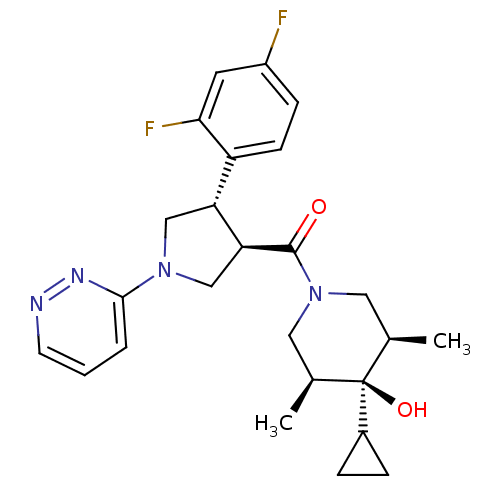
(CHEMBL1204056)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)C1CC1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C25H30F2N4O2/c1-15-11-31(12-16(2)25(15,33)17-5-6-17)24(32)21-14-30(23-4-3-9-28-29-23)13-20(21)19-8-7-18(26)10-22(19)27/h3-4,7-10,15-17,20-21,33H,5-6,11-14H2,1-2H3/t15-,16+,20-,21+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315669
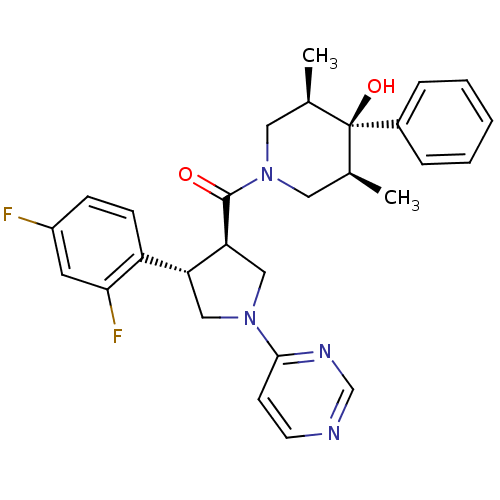
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyrimidin-4-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1ccncn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-13-34(14-19(2)28(18,36)20-6-4-3-5-7-20)27(35)24-16-33(26-10-11-31-17-32-26)15-23(24)22-9-8-21(29)12-25(22)30/h3-12,17-19,23-24,36H,13-16H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315673
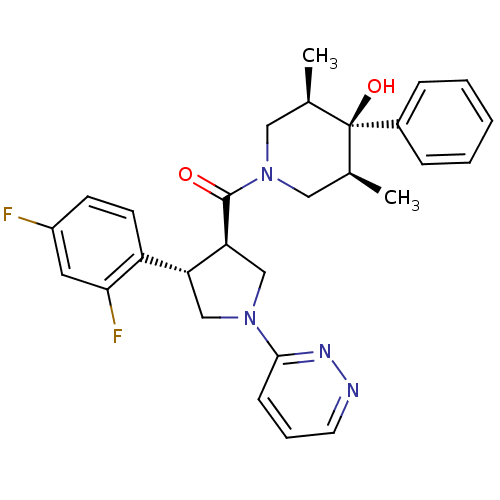
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridazin-3-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-14-34(15-19(2)28(18,36)20-7-4-3-5-8-20)27(35)24-17-33(26-9-6-12-31-32-26)16-23(24)22-11-10-21(29)13-25(22)30/h3-13,18-19,23-24,36H,14-17H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378748
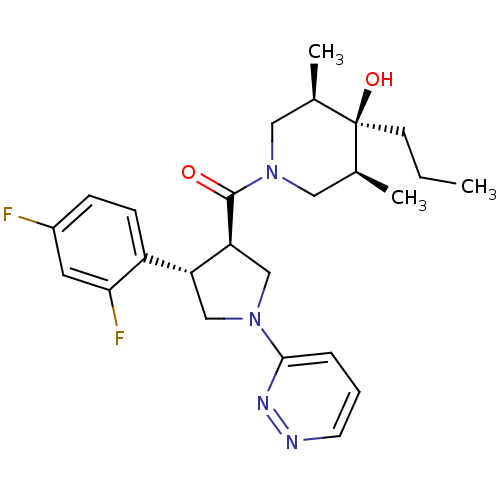
(CHEMBL1204054)Show SMILES CCC[C@]1(O)[C@@H](C)CN(C[C@H]1C)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C25H32F2N4O2/c1-4-9-25(33)16(2)12-31(13-17(25)3)24(32)21-15-30(23-6-5-10-28-29-23)14-20(21)19-8-7-18(26)11-22(19)27/h5-8,10-11,16-17,20-21,33H,4,9,12-15H2,1-3H3/t16-,17+,20-,21+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315673

(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridazin-3-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-14-34(15-19(2)28(18,36)20-7-4-3-5-8-20)27(35)24-17-33(26-9-6-12-31-32-26)16-23(24)22-11-10-21(29)13-25(22)30/h3-13,18-19,23-24,36H,14-17H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315692
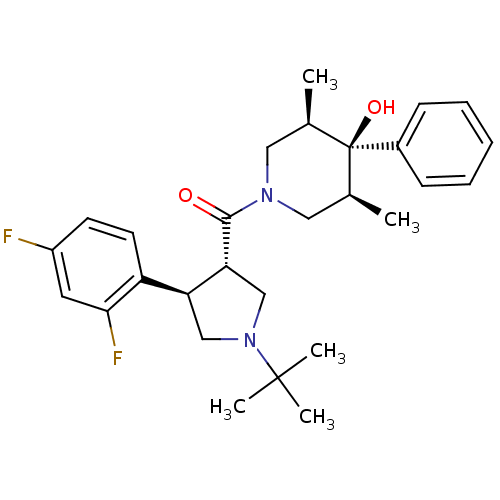
(CHEMBL1090488 | CHEMBL1204059)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H36F2N2O2/c1-18-14-31(15-19(2)28(18,34)20-9-7-6-8-10-20)26(33)24-17-32(27(3,4)5)16-23(24)22-12-11-21(29)13-25(22)30/h6-13,18-19,23-24,34H,14-17H2,1-5H3/t18-,19+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315691
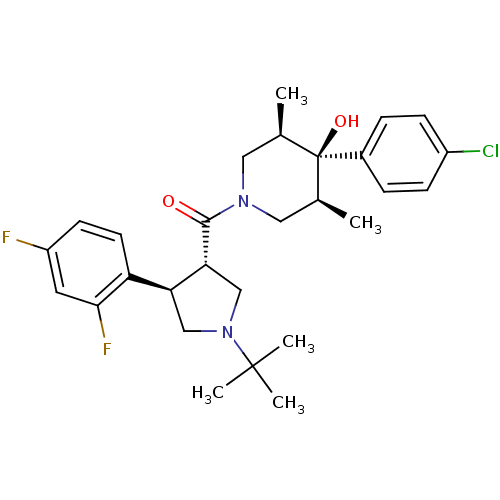
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(Cl)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35ClF2N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305524
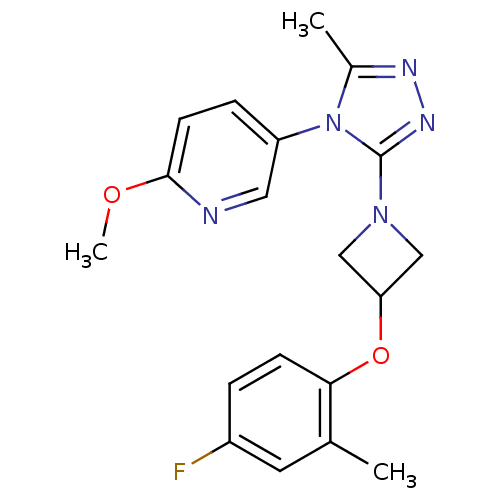
(5-(3-(3-(4-fluoro-2-methylphenoxy)azetidin-1-yl)-5...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1N1CC(C1)Oc1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-12-8-14(20)4-6-17(12)27-16-10-24(11-16)19-23-22-13(2)25(19)15-5-7-18(26-3)21-9-15/h4-9,16H,10-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305517

((R)-5-(3-(3-(4-fluoro-2-methylphenoxy)pyrrolidin-1...)Show SMILES COCc1nnc(N2CC[C@H](C2)Oc2ccc(F)cc2C)n1-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H24FN5O3/c1-14-10-15(22)4-6-18(14)30-17-8-9-26(12-17)21-25-24-19(13-28-2)27(21)16-5-7-20(29-3)23-11-16/h4-7,10-11,17H,8-9,12-13H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471493
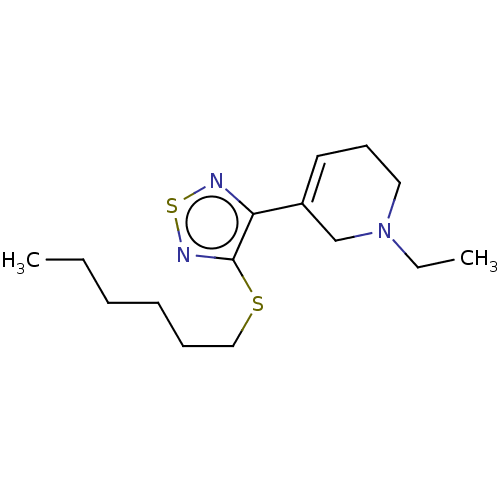
(CHEMBL152919)Show InChI InChI=1S/C15H25N3S2/c1-3-5-6-7-11-19-15-14(16-20-17-15)13-9-8-10-18(4-2)12-13/h9H,3-8,10-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data