 Found 79 hits with Last Name = 'bye' and Initial = 'r'
Found 79 hits with Last Name = 'bye' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-glucosidase MAL62
(Saccharomyces cerevisiae) | BDBM50333465
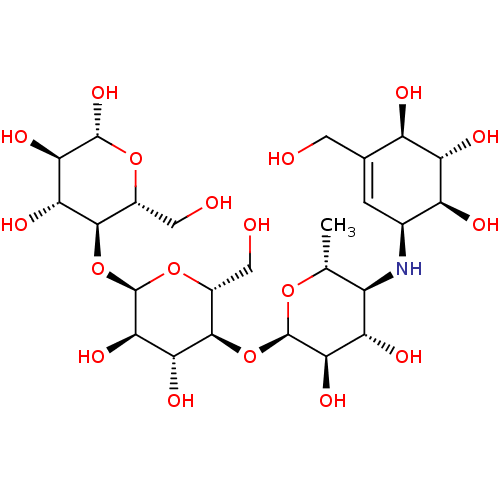
((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...)Show SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)O[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |r,t:37| Show InChI InChI=1S/C25H43NO18/c1-6-11(26-8-2-7(3-27)12(30)15(33)13(8)31)14(32)19(37)24(40-6)43-22-10(5-29)42-25(20(38)17(22)35)44-21-9(4-28)41-23(39)18(36)16(21)34/h2,6,8-39H,3-5H2,1H3/t6-,8+,9-,10-,11-,12-,13+,14+,15+,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-/m1/s1 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Auto£?noma de Me£?xico
Curated by ChEMBL
| Assay Description
Inhibition of yeast alpha-glucosidase assessed as inhibition of p-nitrophenyl-alpha-D-glucopyranoside substrate hydrolysis after 35 mins by spectrosc... |
J Nat Prod 74: 314-20 (2011)
Article DOI: 10.1021/np100447a
BindingDB Entry DOI: 10.7270/Q25D8S53 |
More data for this
Ligand-Target Pair | |
Alpha-glucosidase MAL62
(Saccharomyces cerevisiae) | BDBM50341204
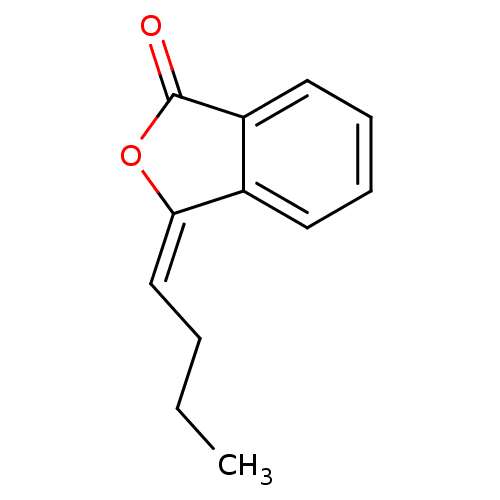
((Z)-3-butylidenephthalide | CHEMBL1765390)Show InChI InChI=1S/C12H12O2/c1-2-3-8-11-9-6-4-5-7-10(9)12(13)14-11/h4-8H,2-3H2,1H3/b11-8+ | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.86E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Auto£?noma de Me£?xico
Curated by ChEMBL
| Assay Description
Inhibition of yeast alpha-glucosidase assessed as inhibition of p-nitrophenyl-alpha-D-glucopyranoside substrate hydrolysis after 35 mins by spectrosc... |
J Nat Prod 74: 314-20 (2011)
Article DOI: 10.1021/np100447a
BindingDB Entry DOI: 10.7270/Q25D8S53 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50358646
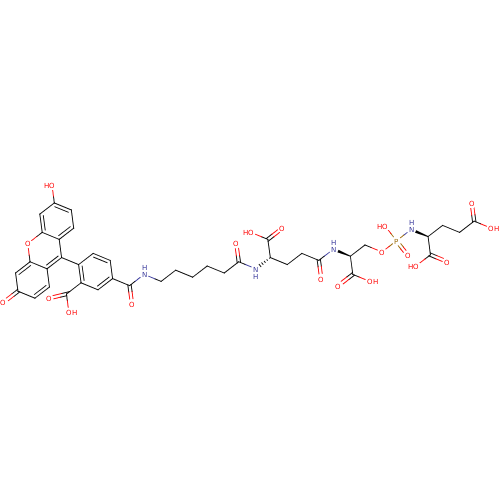
(CHEMBL1921899)Show SMILES OC(=O)CC[C@H](NP(O)(=O)OC[C@H](NC(=O)CC[C@H](NC(=O)CCCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(O)cc2oc2cc(=O)ccc12)C(O)=O)C(O)=O)C(O)=O |r,wD:18.18,12.12,5.5,(26.08,-15.33,;26.07,-13.79,;27.4,-13.02,;24.72,-13.04,;23.39,-13.81,;22.06,-13.06,;20.73,-13.83,;19.39,-13.07,;19.38,-14.61,;18.06,-13.85,;19.38,-11.53,;18.04,-10.77,;18.03,-9.23,;16.69,-8.47,;15.36,-9.24,;15.38,-10.78,;14.02,-8.49,;12.7,-9.26,;11.36,-8.51,;10.03,-9.28,;8.69,-8.52,;8.68,-6.98,;7.36,-9.3,;6.02,-8.54,;4.69,-9.32,;3.36,-8.56,;2.03,-9.34,;.68,-8.58,;-.64,-9.35,;-1.98,-8.6,;-.63,-10.89,;-1.96,-11.68,;-1.95,-13.22,;-.62,-13.97,;.71,-13.2,;.71,-11.66,;2.04,-12.42,;3.39,-13.18,;2.04,-10.88,;-.61,-15.51,;-1.93,-16.3,;-3.27,-15.53,;-4.6,-16.3,;-4.6,-17.85,;-5.93,-18.61,;-3.27,-18.62,;-1.92,-17.84,;-.59,-18.61,;.74,-17.83,;2.07,-18.58,;3.4,-17.81,;4.74,-18.57,;3.39,-16.27,;2.05,-15.51,;.74,-16.28,;11.34,-6.96,;12.68,-6.18,;10.01,-6.2,;19.36,-8.45,;20.7,-9.21,;19.35,-6.91,;22.05,-11.52,;23.38,-10.73,;20.7,-10.75,)| Show InChI InChI=1S/C40H43N4O19P/c45-21-6-9-24-30(17-21)63-31-18-22(46)7-10-25(31)35(24)23-8-5-20(16-26(23)37(52)53)36(51)41-15-3-1-2-4-32(47)42-27(38(54)55)11-13-33(48)43-29(40(58)59)19-62-64(60,61)44-28(39(56)57)12-14-34(49)50/h5-10,16-18,27-29,45H,1-4,11-15,19H2,(H,41,51)(H,42,47)(H,43,48)(H,49,50)(H,52,53)(H,54,55)(H,56,57)(H,58,59)(H2,44,60,61)/t27-,28-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA using N-[4-(phenylazo)-benzoyl]-glutamyl-gamma-glutamic acid as substrate after 15 mins by HPLC analysis |
Bioorg Med Chem Lett 21: 7013-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.115
BindingDB Entry DOI: 10.7270/Q2PG1S5S |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50358645
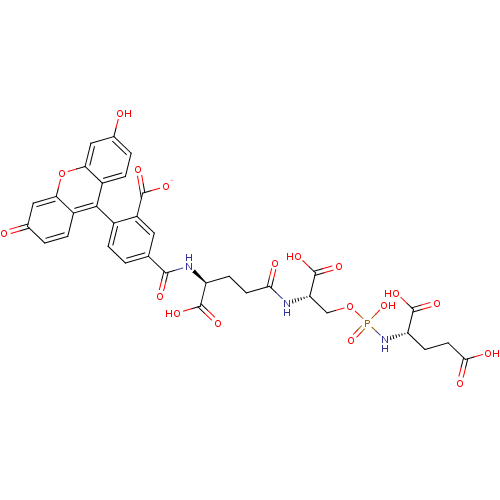
(CHEMBL1921898)Show SMILES OC(=O)CC[C@H](NP(O)(=O)OC[C@H](NC(=O)CC[C@H](NC(=O)c1ccc(c(c1)C([O-])=O)-c1c2ccc(O)cc2oc2cc(=O)ccc12)C(O)=O)C(O)=O)C(O)=O |r,wU:18.18,wD:12.12,5.5,(11.85,-15.93,;11.85,-14.39,;13.19,-13.62,;10.52,-13.62,;9.19,-14.39,;7.85,-13.62,;6.52,-14.39,;5.19,-13.63,;5.17,-15.16,;3.86,-14.4,;5.19,-12.09,;3.86,-11.32,;3.86,-9.78,;2.52,-9.01,;1.19,-9.78,;1.19,-11.32,;-.14,-9.02,;-1.48,-9.79,;-2.81,-9.02,;-2.81,-7.48,;-4.14,-6.71,;-5.47,-7.48,;-4.14,-5.17,;-5.48,-4.4,;-5.48,-2.87,;-4.14,-2.09,;-2.82,-2.86,;-2.81,-4.39,;-1.48,-2.09,;-.15,-2.85,;-2.58,-.99,;-4.14,-.56,;-5.48,.21,;-6.81,-.55,;-8.14,.22,;-8.14,1.76,;-9.47,2.53,;-6.81,2.53,;-5.48,1.77,;-4.14,2.55,;-2.79,1.77,;-1.46,2.54,;-.13,1.77,;1.2,2.54,;-.12,.22,;-1.46,-.56,;-2.8,.22,;-4.14,-9.79,;-5.47,-9.02,;-4.14,-11.33,;5.18,-9.01,;6.52,-9.78,;5.18,-7.47,;7.85,-12.09,;9.18,-11.31,;6.52,-11.31,)| Show InChI InChI=1S/C34H32N3O18P/c38-16-2-5-19-25(12-16)55-26-13-17(39)3-6-20(26)29(19)18-4-1-15(11-21(18)31(44)45)30(43)36-22(32(46)47)7-9-27(40)35-24(34(50)51)14-54-56(52,53)37-23(33(48)49)8-10-28(41)42/h1-6,11-13,22-24,38H,7-10,14H2,(H,35,40)(H,36,43)(H,41,42)(H,44,45)(H,46,47)(H,48,49)(H,50,51)(H2,37,52,53)/p-1/t22-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA using N-[4-(phenylazo)-benzoyl]-glutamyl-gamma-glutamic acid as substrate after 15 mins by HPLC analysis |
Bioorg Med Chem Lett 21: 7013-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.115
BindingDB Entry DOI: 10.7270/Q2PG1S5S |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50358647
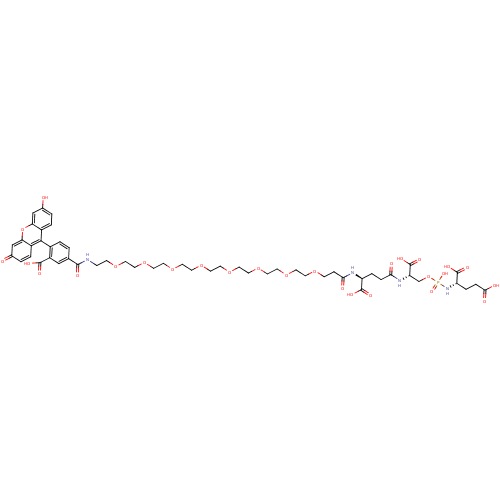
(CHEMBL1921900)Show SMILES OC(=O)CC[C@H](NP(O)(=O)OC[C@H](NC(=O)CC[C@H](NC(=O)CCOCCOCCOCCOCCOCCOCCOCCOCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(O)cc2oc2cc(=O)ccc12)C(O)=O)C(O)=O)C(O)=O |r,wD:18.18,12.12,5.5,(58.79,-21.53,;58.78,-20,;60.12,-19.23,;57.45,-19.23,;56.12,-20,;54.79,-19.23,;53.46,-20,;52.12,-19.23,;53.21,-18.14,;50.79,-20,;52.12,-17.69,;50.79,-16.92,;50.79,-15.39,;49.45,-14.61,;48.13,-15.38,;48.13,-16.92,;46.79,-14.61,;45.47,-15.38,;44.13,-14.61,;42.8,-15.38,;41.47,-14.61,;41.47,-13.08,;40.14,-15.38,;38.8,-14.61,;37.48,-15.38,;36.14,-14.62,;34.81,-15.39,;33.48,-14.62,;32.15,-15.39,;30.81,-14.62,;29.48,-15.39,;28.15,-14.62,;26.82,-15.39,;25.49,-14.62,;24.15,-15.39,;22.82,-14.62,;21.49,-15.39,;20.16,-14.62,;18.82,-15.39,;17.5,-14.62,;16.16,-15.39,;14.83,-14.63,;13.5,-15.39,;12.17,-14.63,;10.83,-15.4,;9.51,-14.63,;8.17,-15.4,;6.84,-14.63,;5.51,-15.4,;4.18,-14.63,;2.84,-15.4,;4.18,-13.09,;2.84,-12.32,;2.84,-10.78,;4.18,-10.02,;5.51,-10.78,;5.51,-12.31,;6.84,-10.01,;8.17,-10.77,;5.73,-8.91,;4.18,-8.47,;2.86,-7.71,;1.53,-8.48,;.19,-7.71,;.2,-6.17,;-1.14,-5.41,;1.52,-5.4,;2.85,-6.16,;4.19,-5.39,;5.53,-6.17,;6.85,-5.4,;8.19,-6.18,;9.52,-5.42,;8.18,-7.72,;6.84,-8.48,;5.52,-7.71,;44.13,-13.07,;45.47,-12.31,;42.8,-12.3,;52.12,-14.62,;53.46,-15.38,;52.12,-13.07,;54.79,-17.68,;56.12,-16.91,;53.45,-16.91,)| Show InChI InChI=1S/C53H69N4O27P/c58-34-2-5-37-43(30-34)84-44-31-35(59)3-6-38(44)48(37)36-4-1-33(29-39(36)50(65)66)49(64)54-12-14-76-16-18-78-20-22-80-24-26-82-28-27-81-25-23-79-21-19-77-17-15-75-13-11-46(61)55-40(51(67)68)7-9-45(60)56-42(53(71)72)32-83-85(73,74)57-41(52(69)70)8-10-47(62)63/h1-6,29-31,40-42,58H,7-28,32H2,(H,54,64)(H,55,61)(H,56,60)(H,62,63)(H,65,66)(H,67,68)(H,69,70)(H,71,72)(H2,57,73,74)/t40-,41-,42-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA using N-[4-(phenylazo)-benzoyl]-glutamyl-gamma-glutamic acid as substrate after 15 mins by HPLC analysis |
Bioorg Med Chem Lett 21: 7013-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.115
BindingDB Entry DOI: 10.7270/Q2PG1S5S |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50358644
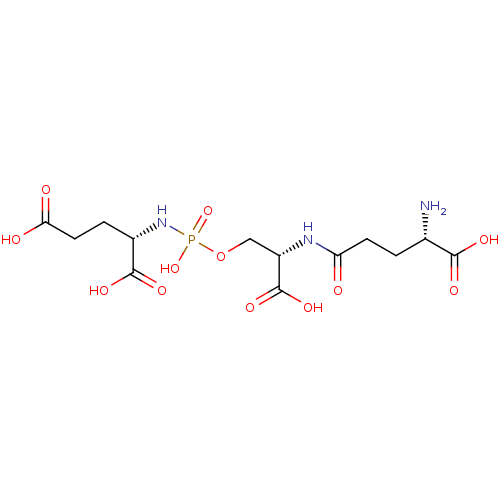
(CHEMBL1921897)Show SMILES N[C@@H](CCC(=O)N[C@@H](COP(O)(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C13H22N3O12P/c14-6(11(20)21)1-3-9(17)15-8(13(24)25)5-28-29(26,27)16-7(12(22)23)2-4-10(18)19/h6-8H,1-5,14H2,(H,15,17)(H,18,19)(H,20,21)(H,22,23)(H,24,25)(H2,16,26,27)/t6-,7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA using N-[4-(phenylazo)-benzoyl]-glutamyl-gamma-glutamic acid as substrate after 15 mins by HPLC analysis |
Bioorg Med Chem Lett 21: 7013-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.115
BindingDB Entry DOI: 10.7270/Q2PG1S5S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477454
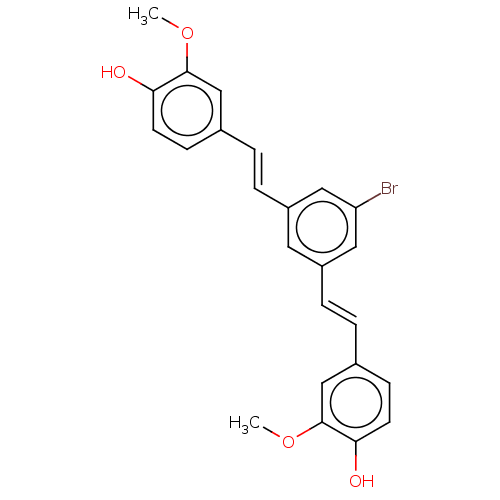
(CHEMBL245688)Show SMILES COc1cc(\C=C\c2cc(Br)cc(\C=C\c3ccc(O)c(OC)c3)c2)ccc1O Show InChI InChI=1S/C24H21BrO4/c1-28-23-14-16(7-9-21(23)26)3-5-18-11-19(13-20(25)12-18)6-4-17-8-10-22(27)24(15-17)29-2/h3-15,26-27H,1-2H3/b5-3+,6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477429
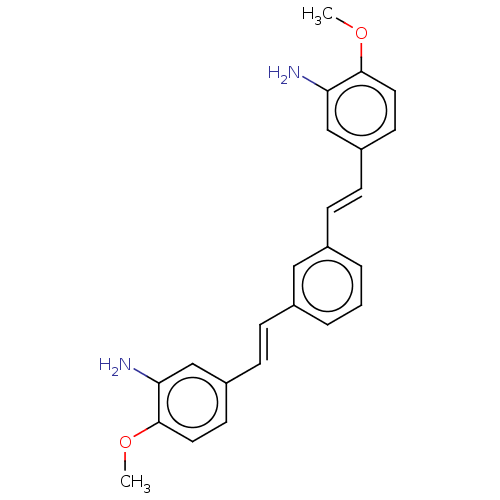
(CHEMBL248132)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(N)c3)c2)cc1N Show InChI InChI=1S/C24H24N2O2/c1-27-23-12-10-19(15-21(23)25)8-6-17-4-3-5-18(14-17)7-9-20-11-13-24(28-2)22(26)16-20/h3-16H,25-26H2,1-2H3/b8-6+,9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477447

(CHEMBL248327)Show SMILES CNc1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(NC)c3)n2)ccc1O Show InChI InChI=1S/C23H23N3O2/c1-24-20-14-16(8-12-22(20)27)6-10-18-4-3-5-19(26-18)11-7-17-9-13-23(28)21(15-17)25-2/h3-15,24-25,27-28H,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477441

(CHEMBL247674)Show SMILES CNc1ccc(\C=C\c2cccc(\C=C\c3ccc(NC)c(OC)c3)n2)cc1OC Show InChI InChI=1S/C25H27N3O2/c1-26-22-14-10-18(16-24(22)29-3)8-12-20-6-5-7-21(28-20)13-9-19-11-15-23(27-2)25(17-19)30-4/h5-17,26-27H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477437
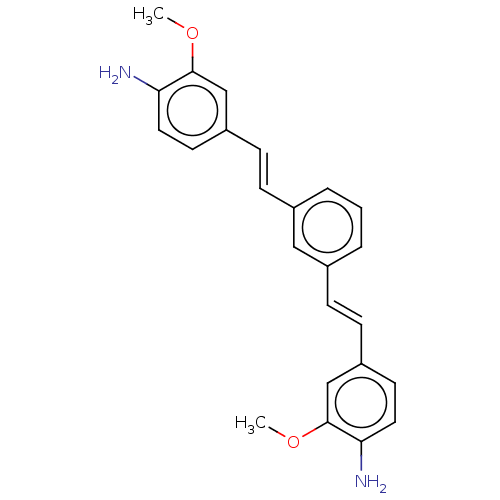
(CHEMBL247930)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N)c(OC)c3)c2)ccc1N Show InChI InChI=1S/C24H24N2O2/c1-27-23-15-19(10-12-21(23)25)8-6-17-4-3-5-18(14-17)7-9-20-11-13-22(26)24(16-20)28-2/h3-16H,25-26H2,1-2H3/b8-6+,9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477456
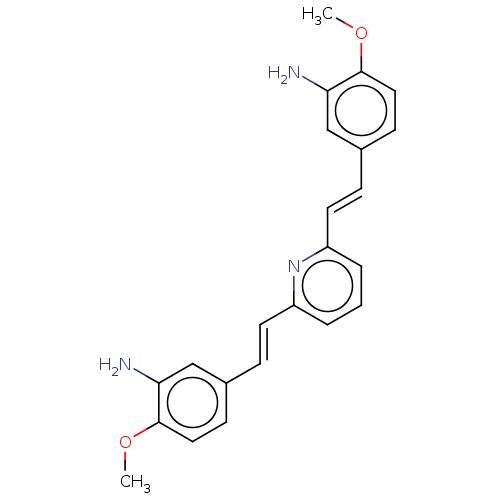
(CHEMBL392878)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(N)c3)n2)cc1N Show InChI InChI=1S/C23H23N3O2/c1-27-22-12-8-16(14-20(22)24)6-10-18-4-3-5-19(26-18)11-7-17-9-13-23(28-2)21(25)15-17/h3-15H,24-25H2,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50105462
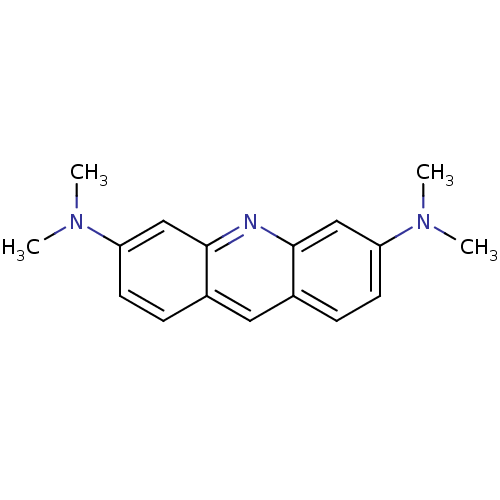
(CHEMBL81880 | N,N,N',N'-Tetramethyl-acridine-3,6-d...)Show InChI InChI=1S/C17H19N3/c1-19(2)14-7-5-12-9-13-6-8-15(20(3)4)11-17(13)18-16(12)10-14/h5-11H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477434

(CHEMBL247931)Show SMILES CNc1ccc(\C=C\c2cccc(\C=C\c3ccc(NC)c(OC)c3)c2)cc1OC Show InChI InChI=1S/C26H28N2O2/c1-27-23-14-12-21(17-25(23)29-3)10-8-19-6-5-7-20(16-19)9-11-22-13-15-24(28-2)26(18-22)30-4/h5-18,27-28H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477428

(CHEMBL248131)Show SMILES CN(C)c1ccc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(O)c3)c2)cc1O Show InChI InChI=1S/C26H28N2O2/c1-27(2)23-14-12-21(17-25(23)29)10-8-19-6-5-7-20(16-19)9-11-22-13-15-24(28(3)4)26(30)18-22/h5-18,29-30H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477439

(CHEMBL247524)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N)c(OC)c3)n2)ccc1N Show InChI InChI=1S/C23H23N3O2/c1-27-22-14-16(8-12-20(22)24)6-10-18-4-3-5-19(26-18)11-7-17-9-13-21(25)23(15-17)28-2/h3-15H,24-25H2,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50067040
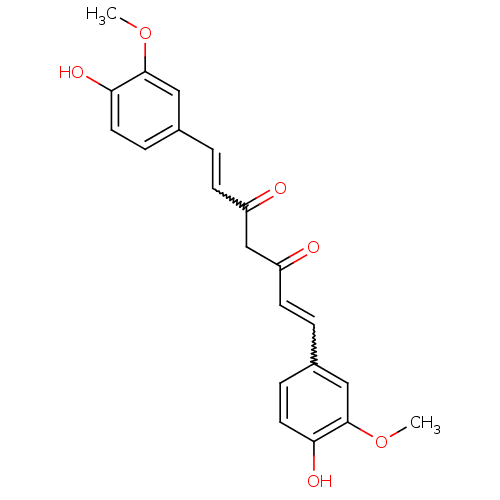
(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477435
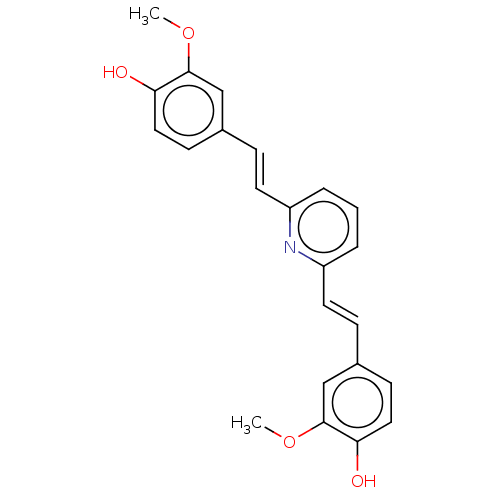
(CHEMBL247328)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(OC)c3)n2)ccc1O Show InChI InChI=1S/C23H21NO4/c1-27-22-14-16(8-12-20(22)25)6-10-18-4-3-5-19(24-18)11-7-17-9-13-21(26)23(15-17)28-2/h3-15,25-26H,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477426

(CHEMBL391205)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(OC)c3)n2)ccc1N(C)C Show InChI InChI=1S/C27H31N3O2/c1-29(2)24-16-12-20(18-26(24)31-5)10-14-22-8-7-9-23(28-22)15-11-21-13-17-25(30(3)4)27(19-21)32-6/h7-19H,1-6H3/b14-10+,15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477450
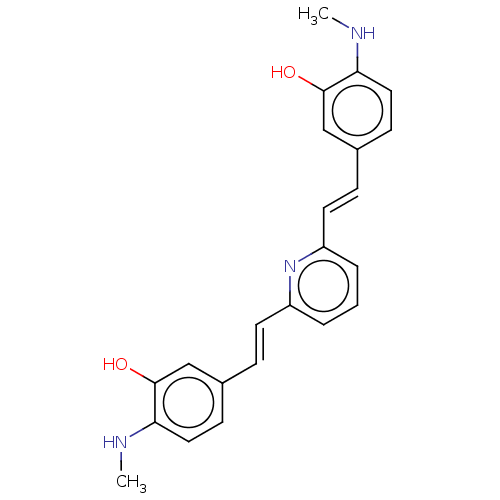
(CHEMBL247725)Show SMILES CNc1ccc(\C=C\c2cccc(\C=C\c3ccc(NC)c(O)c3)n2)cc1O Show InChI InChI=1S/C23H23N3O2/c1-24-20-12-8-16(14-22(20)27)6-10-18-4-3-5-19(26-18)11-7-17-9-13-21(25-2)23(28)15-17/h3-15,24-25,27-28H,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477446
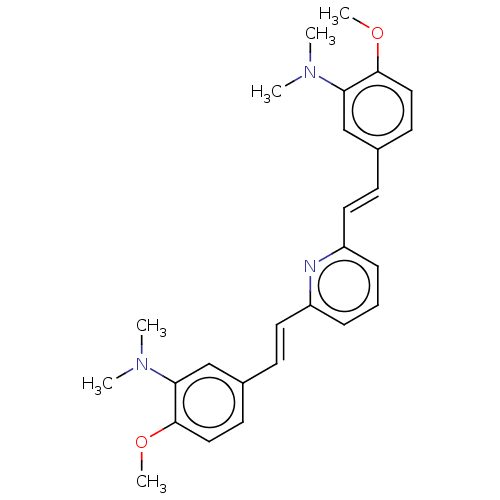
(CHEMBL394713)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(c3)N(C)C)n2)cc1N(C)C Show InChI InChI=1S/C27H31N3O2/c1-29(2)24-18-20(12-16-26(24)31-5)10-14-22-8-7-9-23(28-22)15-11-21-13-17-27(32-6)25(19-21)30(3)4/h7-19H,1-6H3/b14-10+,15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477444
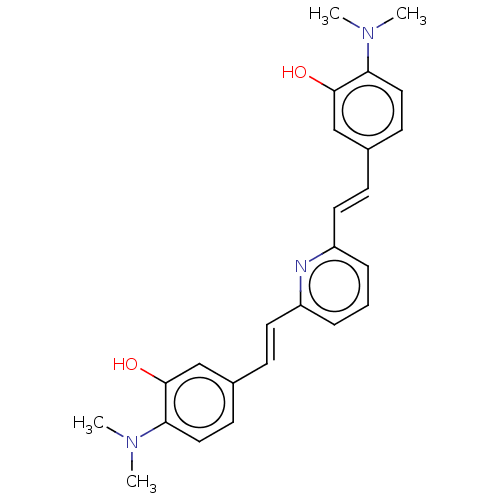
(CHEMBL247927)Show SMILES CN(C)c1ccc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(O)c3)n2)cc1O Show InChI InChI=1S/C25H27N3O2/c1-27(2)22-14-10-18(16-24(22)29)8-12-20-6-5-7-21(26-20)13-9-19-11-15-23(28(3)4)25(30)17-19/h5-17,29-30H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477448
(CHEMBL247932)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(OC)c3)c2)ccc1N(C)C Show InChI InChI=1S/C28H32N2O2/c1-29(2)25-16-14-23(19-27(25)31-5)12-10-21-8-7-9-22(18-21)11-13-24-15-17-26(30(3)4)28(20-24)32-6/h7-20H,1-6H3/b12-10+,13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477448

(CHEMBL247932)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(OC)c3)c2)ccc1N(C)C Show InChI InChI=1S/C28H32N2O2/c1-29(2)25-16-14-23(19-27(25)31-5)12-10-21-8-7-9-22(18-21)11-13-24-15-17-26(30(3)4)28(20-24)32-6/h7-20H,1-6H3/b12-10+,13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta fibril aggregation (unknown origin) by thioflavin T fluorescence method |
Bioorg Med Chem Lett 23: 3467-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.104
BindingDB Entry DOI: 10.7270/Q200050P |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477438

(CHEMBL248122)Show SMILES CNc1cc(\C=C\c2cccc(\C=C\c3ccc(OC)c(NC)c3)n2)ccc1OC Show InChI InChI=1S/C25H27N3O2/c1-26-22-16-18(10-14-24(22)29-3)8-12-20-6-5-7-21(28-20)13-9-19-11-15-25(30-4)23(17-19)27-2/h5-17,26-27H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477436
(CHEMBL247530)Show SMILES COc1cc(\C=C\c2cc(Cl)cc(\C=C\c3ccc(O)c(OC)c3)n2)ccc1O Show InChI InChI=1S/C23H20ClNO4/c1-28-22-11-15(5-9-20(22)26)3-7-18-13-17(24)14-19(25-18)8-4-16-6-10-21(27)23(12-16)29-2/h3-14,26-27H,1-2H3/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477455
(CHEMBL247929)Show SMILES COc1cc(\C=C\c2cc(Br)cc(\C=C\c3ccc(N)c(OC)c3)n2)ccc1N Show InChI InChI=1S/C23H22BrN3O2/c1-28-22-11-15(5-9-20(22)25)3-7-18-13-17(24)14-19(27-18)8-4-16-6-10-21(26)23(12-16)29-2/h3-14H,25-26H2,1-2H3/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477431
(CHEMBL427878)Show SMILES COc1cc(\C=C\c2cc(Br)cc(\C=C\c3ccc(O)c(OC)c3)n2)ccc1O Show InChI InChI=1S/C23H20BrNO4/c1-28-22-11-15(5-9-20(22)26)3-7-18-13-17(24)14-19(25-18)8-4-16-6-10-21(27)23(12-16)29-2/h3-14,26-27H,1-2H3/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477453
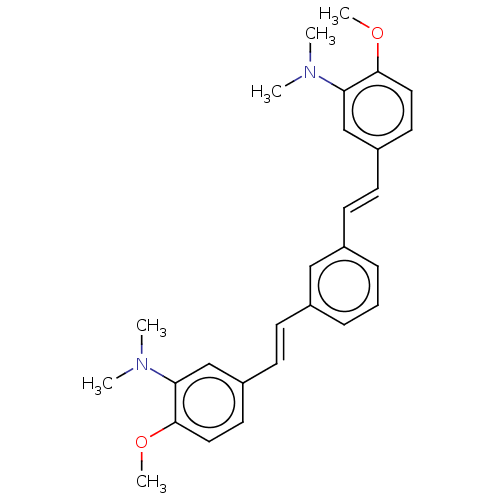
(CHEMBL248337)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(c3)N(C)C)c2)cc1N(C)C Show InChI InChI=1S/C28H32N2O2/c1-29(2)25-19-23(14-16-27(25)31-5)12-10-21-8-7-9-22(18-21)11-13-24-15-17-28(32-6)26(20-24)30(3)4/h7-20H,1-6H3/b12-10+,13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477433

(CHEMBL248133)Show SMILES CNc1cc(\C=C\c2cccc(\C=C\c3ccc(OC)c(NC)c3)c2)ccc1OC Show InChI InChI=1S/C26H28N2O2/c1-27-23-17-21(12-14-25(23)29-3)10-8-19-6-5-7-20(16-19)9-11-22-13-15-26(30-4)24(18-22)28-2/h5-18,27-28H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477451
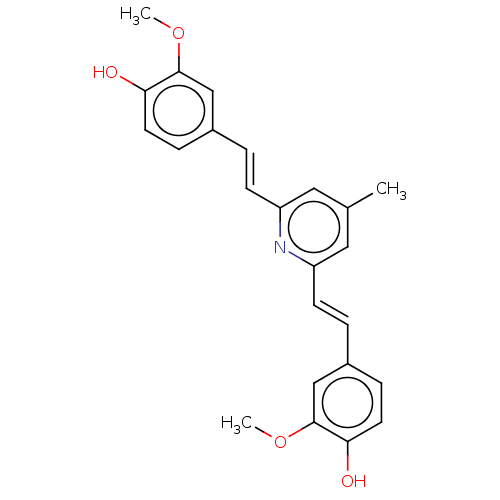
(CHEMBL247727)Show SMILES COc1cc(\C=C\c2cc(C)cc(\C=C\c3ccc(O)c(OC)c3)n2)ccc1O Show InChI InChI=1S/C24H23NO4/c1-16-12-19(8-4-17-6-10-21(26)23(14-17)28-2)25-20(13-16)9-5-18-7-11-22(27)24(15-18)29-3/h4-15,26-27H,1-3H3/b8-4+,9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477445
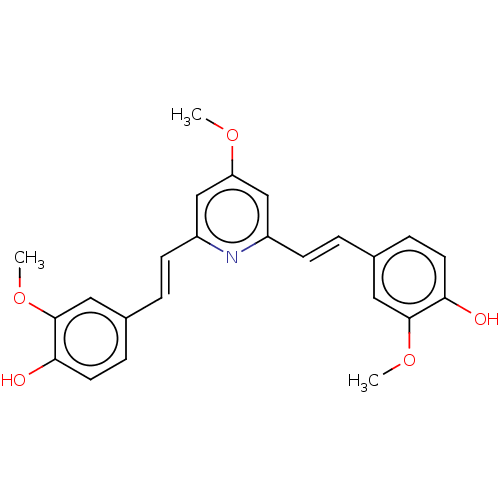
(CHEMBL247726)Show SMILES COc1cc(\C=C\c2ccc(O)c(OC)c2)nc(\C=C\c2ccc(O)c(OC)c2)c1 Show InChI InChI=1S/C24H23NO5/c1-28-20-14-18(8-4-16-6-10-21(26)23(12-16)29-2)25-19(15-20)9-5-17-7-11-22(27)24(13-17)30-3/h4-15,26-27H,1-3H3/b8-4+,9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477452
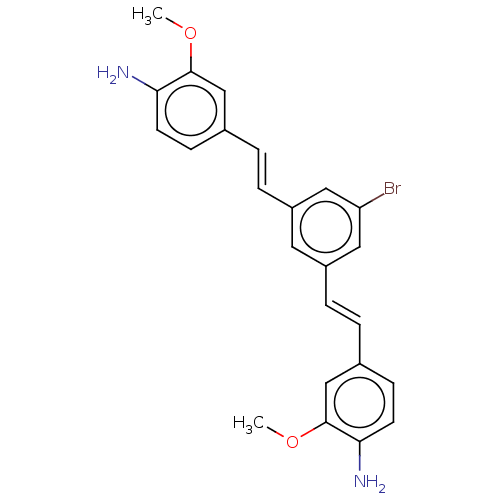
(CHEMBL396917)Show SMILES COc1cc(\C=C\c2cc(Br)cc(\C=C\c3ccc(N)c(OC)c3)c2)ccc1N Show InChI InChI=1S/C24H23BrN2O2/c1-28-23-14-16(7-9-21(23)26)3-5-18-11-19(13-20(25)12-18)6-4-17-8-10-22(27)24(15-17)29-2/h3-15H,26-27H2,1-2H3/b5-3+,6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477432

(CHEMBL247728)Show SMILES COc1cc(\C=C\c2cc(cc(\C=C\c3ccc(O)c(OC)c3)n2)N(C)C)ccc1O Show InChI InChI=1S/C25H26N2O4/c1-27(2)21-15-19(9-5-17-7-11-22(28)24(13-17)30-3)26-20(16-21)10-6-18-8-12-23(29)25(14-18)31-4/h5-16,28-29H,1-4H3/b9-5+,10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477443

(CHEMBL427877)Show SMILES CN(C)c1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(c3)N(C)C)n2)ccc1O Show InChI InChI=1S/C25H27N3O2/c1-27(2)22-16-18(10-14-24(22)29)8-12-20-6-5-7-21(26-20)13-9-19-11-15-25(30)23(17-19)28(3)4/h5-17,29-30H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477425
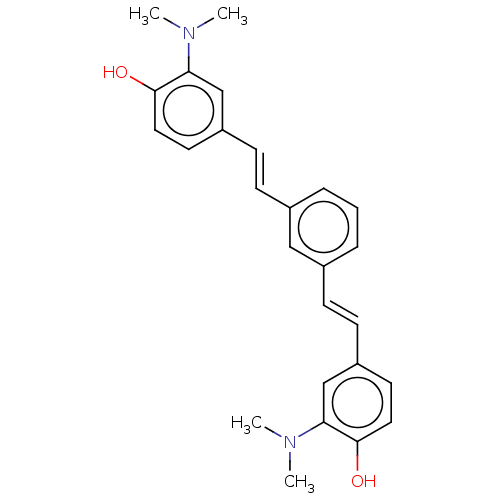
(CHEMBL248338)Show SMILES CN(C)c1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(c3)N(C)C)c2)ccc1O Show InChI InChI=1S/C26H28N2O2/c1-27(2)23-17-21(12-14-25(23)29)10-8-19-6-5-7-20(16-19)9-11-22-13-15-26(30)24(18-22)28(3)4/h5-18,29-30H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50204084
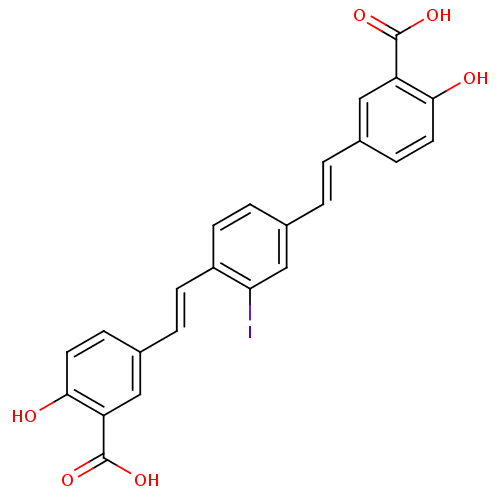
(5-((E)-2-{4-[(E)-2-(3-carboxy-4-hydroxyphenyl)viny...)Show SMILES OC(=O)c1cc(\C=C\c2ccc(\C=C\c3ccc(O)c(c3)C(O)=O)c(I)c2)ccc1O Show InChI InChI=1S/C24H17IO6/c25-20-13-16(2-1-14-5-9-21(26)18(11-14)23(28)29)4-8-17(20)7-3-15-6-10-22(27)19(12-15)24(30)31/h1-13,26-27H,(H,28,29)(H,30,31)/b2-1+,7-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477442
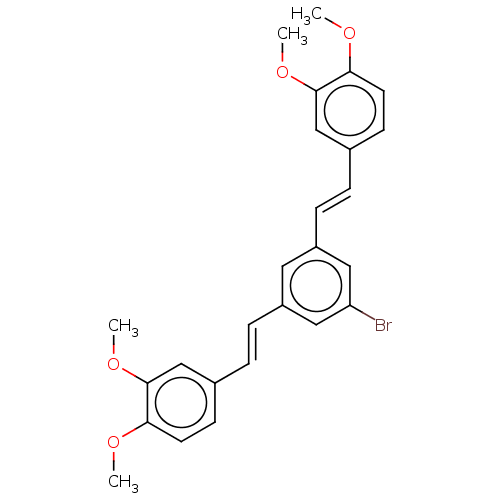
(CHEMBL245476)Show SMILES COc1ccc(\C=C\c2cc(Br)cc(\C=C\c3ccc(OC)c(OC)c3)c2)cc1OC Show InChI InChI=1S/C26H25BrO4/c1-28-23-11-9-18(16-25(23)30-3)5-7-20-13-21(15-22(27)14-20)8-6-19-10-12-24(29-2)26(17-19)31-4/h5-17H,1-4H3/b7-5+,8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477449
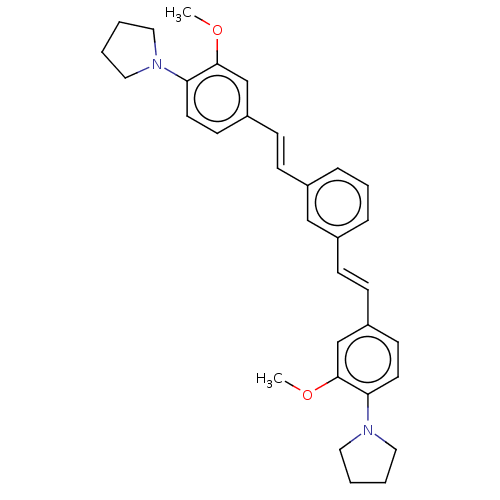
(CHEMBL248339)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N4CCCC4)c(OC)c3)c2)ccc1N1CCCC1 Show InChI InChI=1S/C32H36N2O2/c1-35-31-23-27(14-16-29(31)33-18-3-4-19-33)12-10-25-8-7-9-26(22-25)11-13-28-15-17-30(32(24-28)36-2)34-20-5-6-21-34/h7-17,22-24H,3-6,18-21H2,1-2H3/b12-10+,13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477440
(CHEMBL245478)Show SMILES CN(C)c1ccc(\C=C\c2cc(Br)cc(\C=C\c3ccc(cc3)N(C)C)c2)cc1 Show InChI InChI=1S/C26H27BrN2/c1-28(2)25-13-9-20(10-14-25)5-7-22-17-23(19-24(27)18-22)8-6-21-11-15-26(16-12-21)29(3)4/h5-19H,1-4H3/b7-5+,8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477430
(CHEMBL245477)Show SMILES CN(C)c1ccc(\C=C\c2cccc(\C=C\c3ccc(cc3)N(C)C)c2)cc1 Show InChI InChI=1S/C26H28N2/c1-27(2)25-16-12-21(13-17-25)8-10-23-6-5-7-24(20-23)11-9-22-14-18-26(19-15-22)28(3)4/h5-20H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477427
(CHEMBL245479)Show SMILES CN(C)c1ccc(\C=C\c2cc(I)cc(\C=C\c3ccc(cc3)N(C)C)c2)cc1 Show InChI InChI=1S/C26H27IN2/c1-28(2)25-13-9-20(10-14-25)5-7-22-17-23(19-24(27)18-22)8-6-21-11-15-26(16-12-21)29(3)4/h5-19H,1-4H3/b7-5+,8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Alpha-xylosidase
(Blautia obeum) | BDBM50412283
(CHEMBL476314)Show InChI InChI=1S/C16H12O7/c1-22-13-6-12-14(16(21)15(13)20)10(19)5-11(23-12)7-2-3-8(17)9(18)4-7/h2-6,17-18,20-21H,1H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Ruminococcus obeum ATCC 29174 alpha-glucosidase expressed in Escherichia coli BL21(DE3) using p-nitrophenyl-alpha-D-glucopy... |
J Nat Prod 80: 1584-1593 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00155
BindingDB Entry DOI: 10.7270/Q2571FH2 |
More data for this
Ligand-Target Pair | |
Alpha-xylosidase
(Blautia obeum) | BDBM50270473
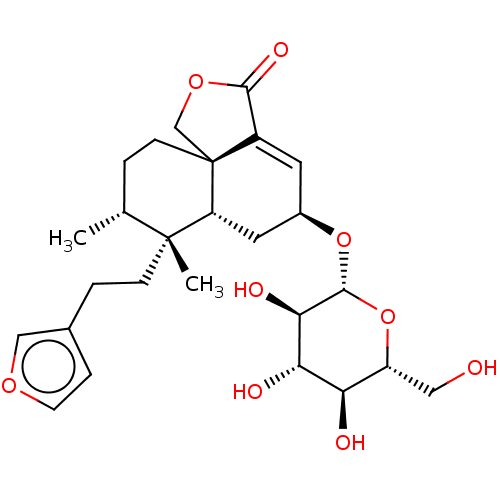
(CHEMBL4127208)Show SMILES [H][C@@]1(C[C@@]2([H])[C@@]3(COC(=O)C3=C1)CC[C@@H](C)[C@@]2(C)CCc1ccoc1)O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r,c:11| Show InChI InChI=1S/C26H36O9/c1-14-3-7-26-13-33-23(31)17(26)9-16(34-24-22(30)21(29)20(28)18(11-27)35-24)10-19(26)25(14,2)6-4-15-5-8-32-12-15/h5,8-9,12,14,16,18-22,24,27-30H,3-4,6-7,10-11,13H2,1-2H3/t14-,16-,18-,19-,20-,21+,22-,24-,25-,26-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Ruminococcus obeum ATCC 29174 alpha-glucosidase expressed in Escherichia coli BL21(DE3) using p-nitrophenyl-alpha-D-glucopy... |
J Nat Prod 80: 1584-1593 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00155
BindingDB Entry DOI: 10.7270/Q2571FH2 |
More data for this
Ligand-Target Pair | |
Alpha-glucosidase MAL62
(Saccharomyces cerevisiae) | BDBM50333465

((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...)Show SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)O[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |r,t:37| Show InChI InChI=1S/C25H43NO18/c1-6-11(26-8-2-7(3-27)12(30)15(33)13(8)31)14(32)19(37)24(40-6)43-22-10(5-29)42-25(20(38)17(22)35)44-21-9(4-28)41-23(39)18(36)16(21)34/h2,6,8-39H,3-5H2,1H3/t6-,8+,9-,10-,11-,12-,13+,14+,15+,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-/m1/s1 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Auto£?noma de Me£?xico
Curated by ChEMBL
| Assay Description
Inhibition of yeast alpha-glucosidase assessed as inhibition of p-nitrophenyl-alpha-D-glucopyranoside substrate hydrolysis after 35 mins by spectrosc... |
J Nat Prod 74: 314-20 (2011)
Article DOI: 10.1021/np100447a
BindingDB Entry DOI: 10.7270/Q25D8S53 |
More data for this
Ligand-Target Pair | |
Alpha-xylosidase
(Blautia obeum) | BDBM23406

((3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5...)Show SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)OC(O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |t:37| Show InChI InChI=1S/C25H43NO18/c1-6-11(26-8-2-7(3-27)12(30)15(33)13(8)31)14(32)19(37)24(40-6)43-22-10(5-29)42-25(20(38)17(22)35)44-21-9(4-28)41-23(39)18(36)16(21)34/h2,6,8-39H,3-5H2,1H3/t6-,8+,9-,10-,11-,12-,13+,14+,15+,16-,17-,18-,19-,20-,21-,22-,23?,24-,25-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Ruminococcus obeum ATCC 29174 alpha-glucosidase expressed in Escherichia coli BL21(DE3) using p-nitrophenyl-alpha-D-glucopy... |
J Nat Prod 80: 1584-1593 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00155
BindingDB Entry DOI: 10.7270/Q2571FH2 |
More data for this
Ligand-Target Pair | |
Alpha-glucosidase MAL62
(Saccharomyces cerevisiae) | BDBM50341204

((Z)-3-butylidenephthalide | CHEMBL1765390)Show InChI InChI=1S/C12H12O2/c1-2-3-8-11-9-6-4-5-7-10(9)12(13)14-11/h4-8H,2-3H2,1H3/b11-8+ | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.35E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Auto£?noma de Me£?xico
Curated by ChEMBL
| Assay Description
Inhibition of yeast alpha-glucosidase assessed as inhibition of p-nitrophenyl-alpha-D-glucopyranoside substrate hydrolysis after 35 mins by spectrosc... |
J Nat Prod 74: 314-20 (2011)
Article DOI: 10.1021/np100447a
BindingDB Entry DOI: 10.7270/Q25D8S53 |
More data for this
Ligand-Target Pair | |
Nuclear factor erythroid 2-related factor 2
(Homo sapiens (Human)) | BDBM50504656
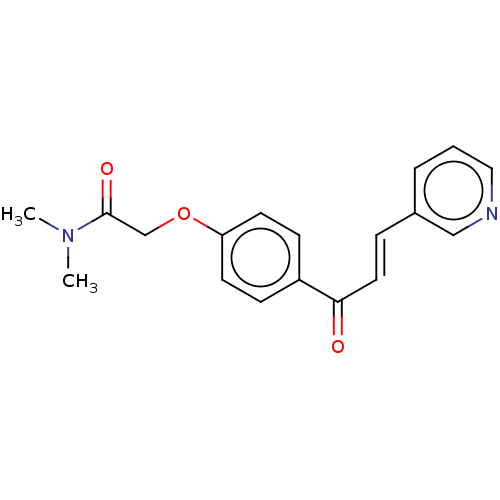
(CHEMBL1549549)Show InChI InChI=1S/C18H18N2O3/c1-20(2)18(22)13-23-16-8-6-15(7-9-16)17(21)10-5-14-4-3-11-19-12-14/h3-12H,13H2,1-2H3/b10-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Activation of NRF2 in human U2OS cells co-expressing Keap1 (unknown origin) assessed as induction of NRF2 translocation to nucleus incubated for 6 hr... |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111777
BindingDB Entry DOI: 10.7270/Q26976TG |
More data for this
Ligand-Target Pair | |
Nuclear factor erythroid 2-related factor 2
(Homo sapiens (Human)) | BDBM50504657
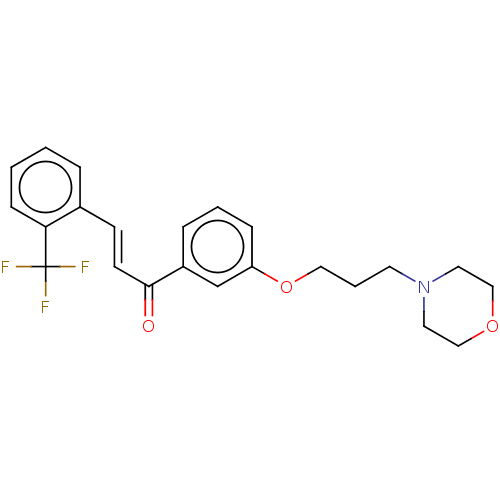
(CHEMBL4577531)Show SMILES Cl.FC(F)(F)c1ccccc1\C=C\C(=O)c1cccc(OCCCN2CCOCC2)c1 Show InChI InChI=1S/C23H24F3NO3/c24-23(25,26)21-8-2-1-5-18(21)9-10-22(28)19-6-3-7-20(17-19)30-14-4-11-27-12-15-29-16-13-27/h1-3,5-10,17H,4,11-16H2/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Activation of NRF2 in human U2OS cells co-expressing Keap1 (unknown origin) assessed as induction of NRF2 translocation to nucleus incubated for 6 hr... |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111777
BindingDB Entry DOI: 10.7270/Q26976TG |
More data for this
Ligand-Target Pair | |
Nuclear factor erythroid 2-related factor 2
(Homo sapiens (Human)) | BDBM50504658

(CHEMBL4564810)Show SMILES Cl.Clc1ccccc1\C=C\C(=O)c1cccc(OCCCN2CCOCC2)c1 Show InChI InChI=1S/C22H24ClNO3/c23-21-8-2-1-5-18(21)9-10-22(25)19-6-3-7-20(17-19)27-14-4-11-24-12-15-26-16-13-24/h1-3,5-10,17H,4,11-16H2/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.84E+3 | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Activation of NRF2 in human U2OS cells co-expressing Keap1 (unknown origin) assessed as induction of NRF2 translocation to nucleus incubated for 6 hr... |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111777
BindingDB Entry DOI: 10.7270/Q26976TG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data