

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM29957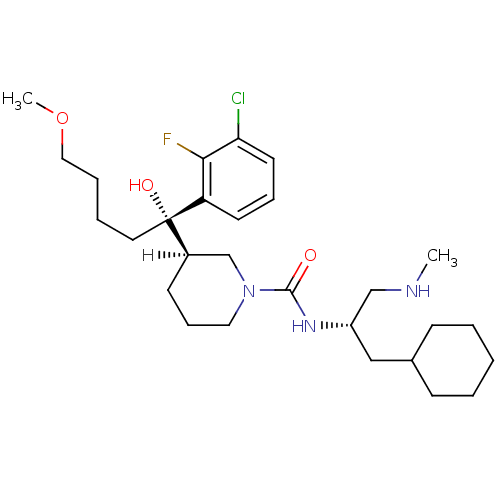 (piperidine-1-carboxamide, 21t) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals | Assay Description The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... | Bioorg Med Chem Lett 19: 3541-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.140 BindingDB Entry DOI: 10.7270/Q2D21VXV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM29950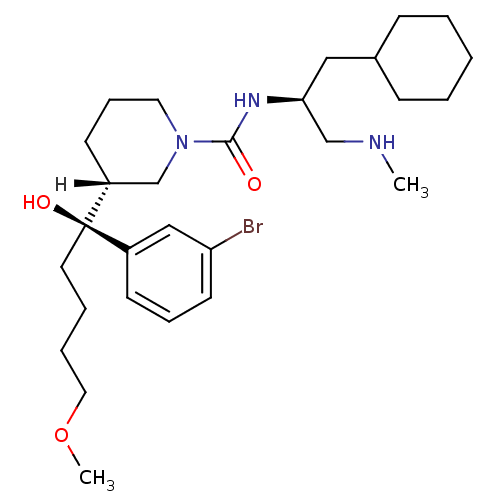 (piperidine-1-carboxamide, 21m) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Vitae Pharmaceuticals | Assay Description The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... | Bioorg Med Chem Lett 19: 3541-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.140 BindingDB Entry DOI: 10.7270/Q2D21VXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM29956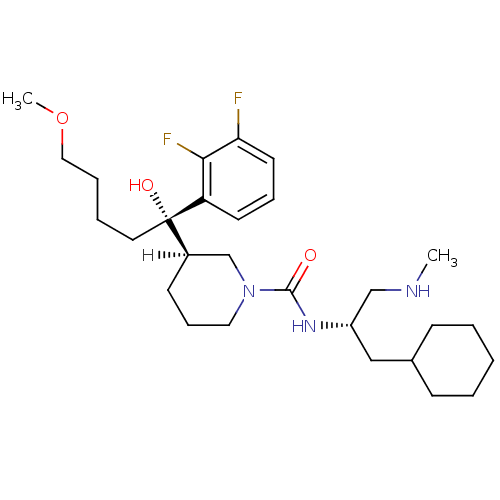 (piperidine-1-carboxamide, 21s) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals | Assay Description The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... | Bioorg Med Chem Lett 19: 3541-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.140 BindingDB Entry DOI: 10.7270/Q2D21VXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50382333 (CHEMBL2023123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305450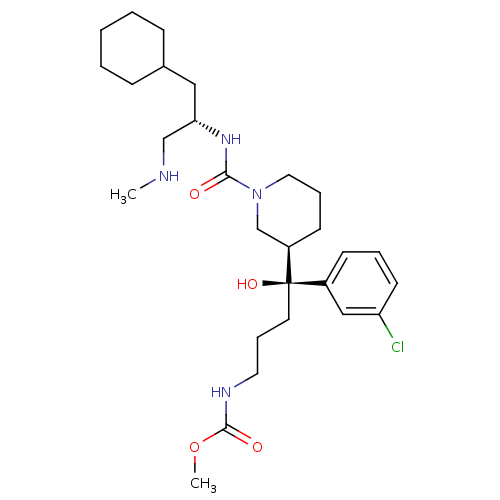 (CHEMBL592763 | methyl (S)-4-(3-chlorophenyl)-4-((R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305465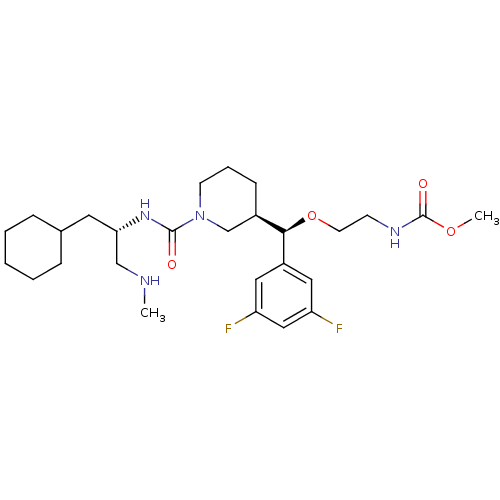 (CHEMBL589647 | methyl 2-((R)-((R)-1-((S)-1-cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50382335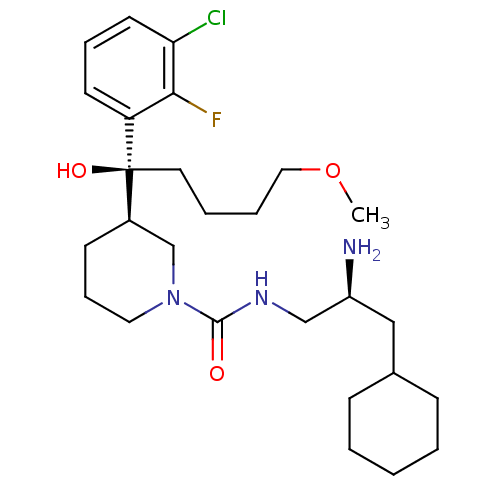 (CHEMBL2024249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50382334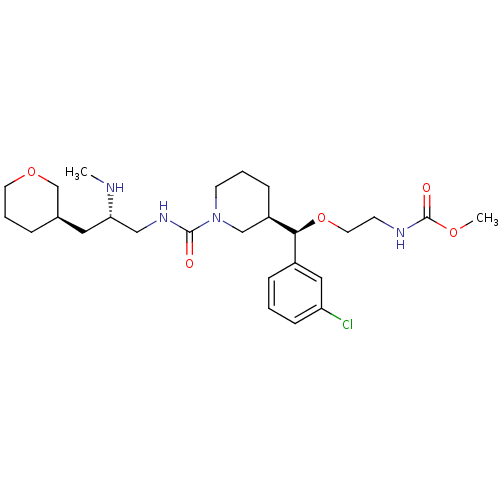 (CHEMBL1276678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50382333 (CHEMBL2023123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353392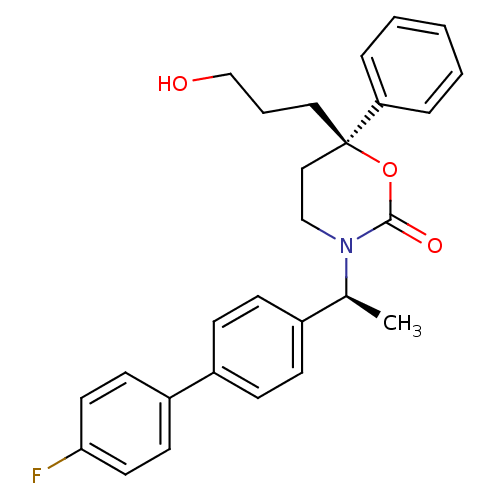 (CHEMBL1829761 | US8575157, 197 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of 11 beta-HSD1 in differentiated human adipocytes assessed as conversion of [3H]-cortisone to [3H]-cortisol after 10 mins by HPLC | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253406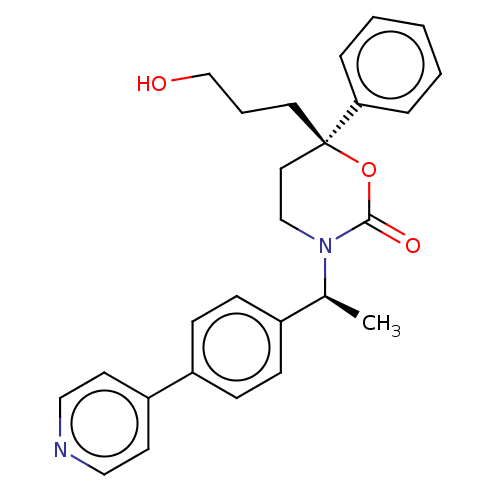 (CHEMBL4101787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50382331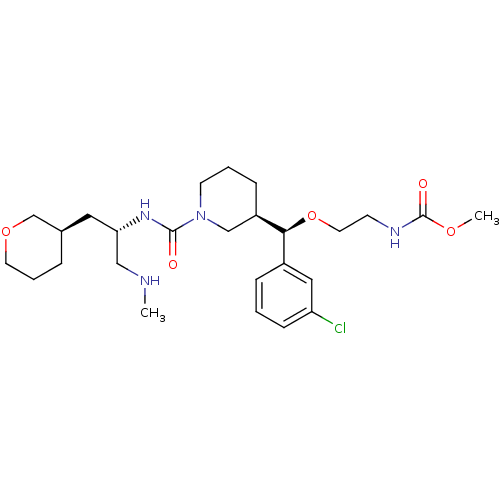 (CHEMBL2024248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM29949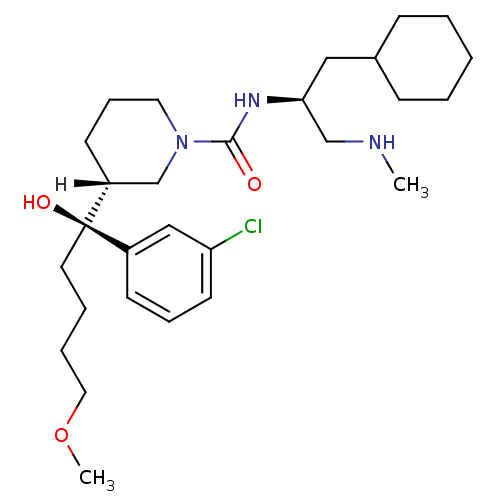 (piperidine-1-carboxamide, 21l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Vitae Pharmaceuticals | Assay Description The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... | Bioorg Med Chem Lett 19: 3541-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.140 BindingDB Entry DOI: 10.7270/Q2D21VXV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50382334 (CHEMBL1276678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM29949 (piperidine-1-carboxamide, 21l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305454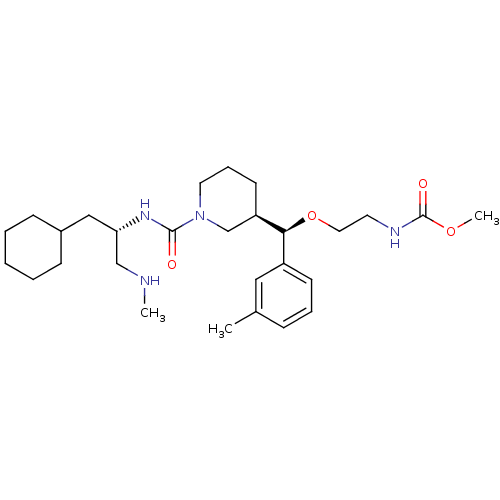 (CHEMBL591342 | methyl 2-((R)-((R)-1-((S)-1-cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305452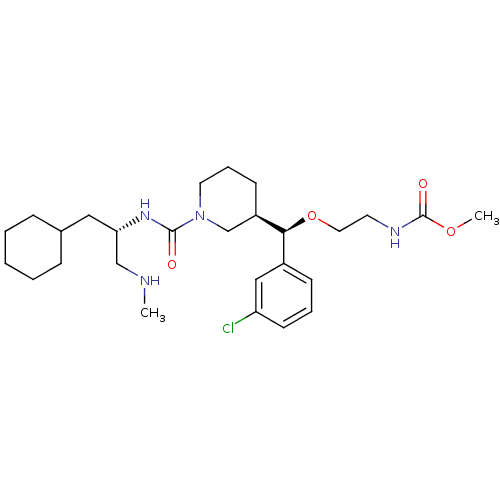 (CHEMBL591578 | methyl 2-((R)-(3-chlorophenyl)((R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305456 (CHEMBL605408 | methyl 2-((R)-((R)-1-((S)-1-amino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50382331 (CHEMBL2024248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of renin in human plasma assessed as formation of angiotensin1 product after 90 mins by competitive radioimmunoassay | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305452 (CHEMBL591578 | methyl 2-((R)-(3-chlorophenyl)((R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253514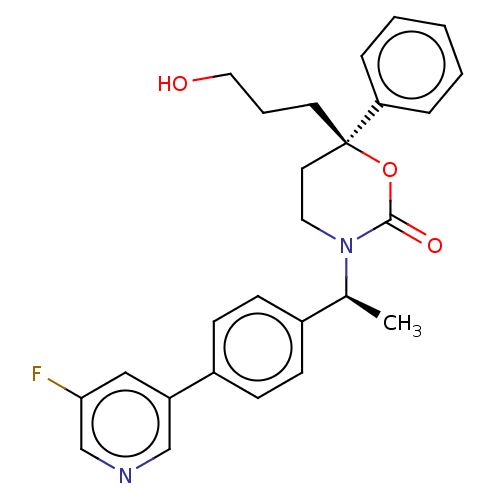 (CHEMBL4075869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253514 (CHEMBL4075869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals | Assay Description The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... | Bioorg Med Chem Lett 19: 3541-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.140 BindingDB Entry DOI: 10.7270/Q2D21VXV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM29949 (piperidine-1-carboxamide, 21l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
USA. Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using of DABCYL-c-Abu-IleHis-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 60 to 360 mins by fluores... | Bioorg Med Chem Lett 21: 4836-43 (2011) Article DOI: 10.1016/j.bmcl.2011.06.043 BindingDB Entry DOI: 10.7270/Q25M663G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353390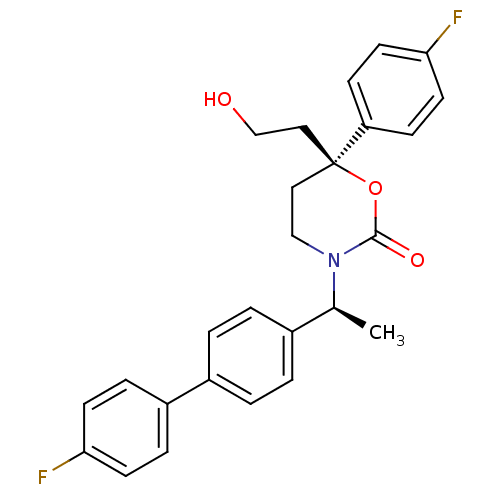 (CHEMBL1829768 | US8575157, 193 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol after 1 hr by scintillation proximity... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50306433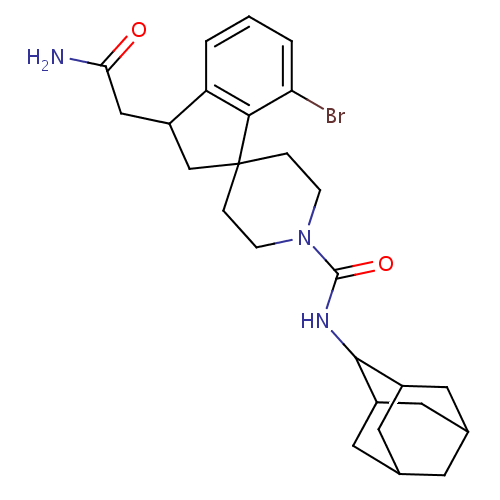 (CHEMBL601211 | N-(adamantan-2-yl)-7-bromo-3-(carba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol by SPA | Bioorg Med Chem Lett 20: 881-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.082 BindingDB Entry DOI: 10.7270/Q2KK9BWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353407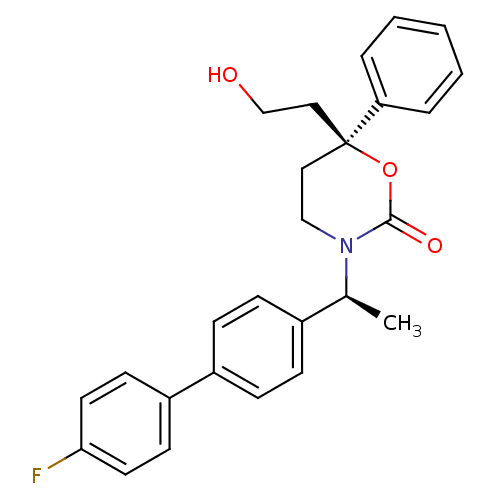 (CHEMBL1829760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol after 1 hr by scintillation proximity... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305466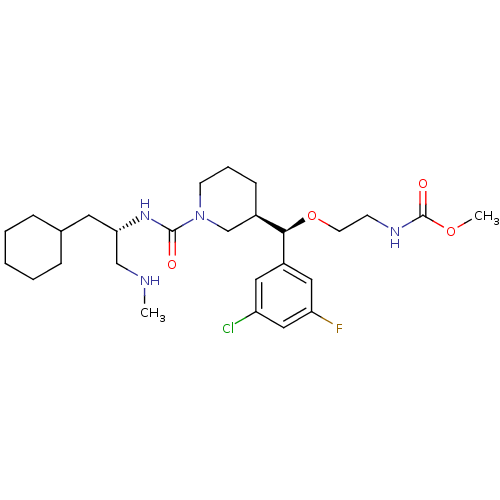 (CHEMBL592765 | methyl 2-((R)-(3-chloro-5-fluorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50382331 (CHEMBL2024248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305457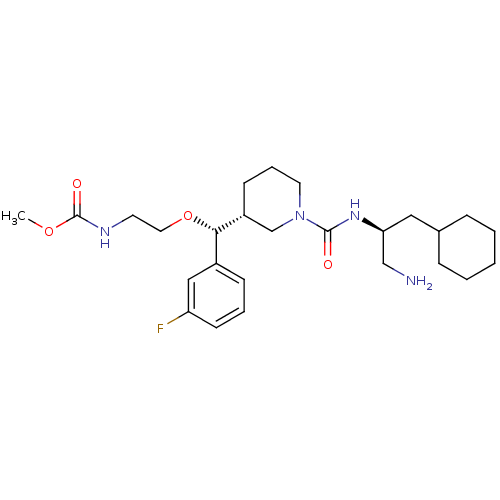 (CHEMBL591340 | methyl 2-((R)-((R)-1-((S)-1-amino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305453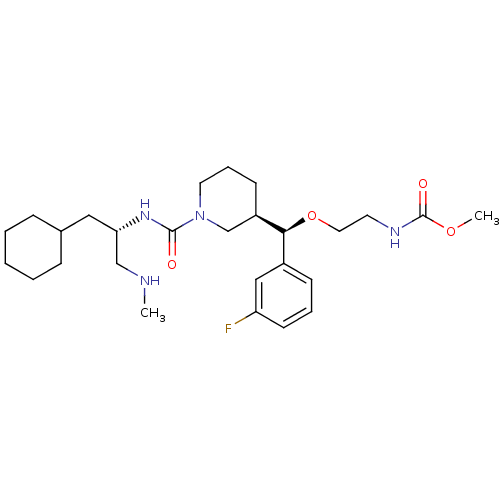 (CHEMBL592762 | methyl 2-((R)-((R)-1-((S)-1-cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253513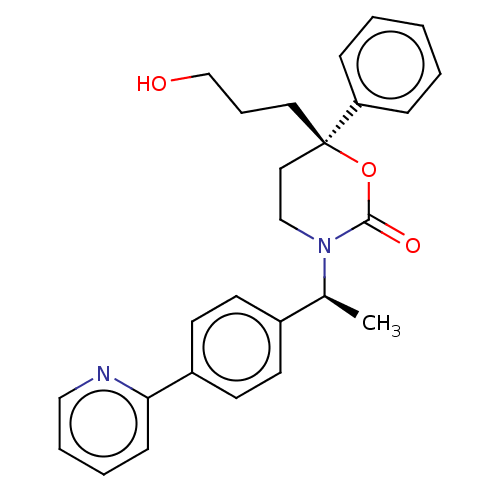 (CHEMBL4060843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253398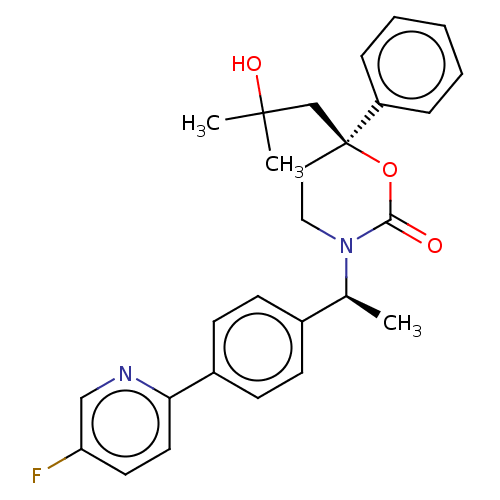 (CHEMBL4096179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253406 (CHEMBL4101787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253336 (CHEMBL4069717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353392 (CHEMBL1829761 | US8575157, 197 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol after 1 hr by scintillation proximity... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195118 (US10336717, Compound 178 | US9212153, 178,Ex. 139) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353405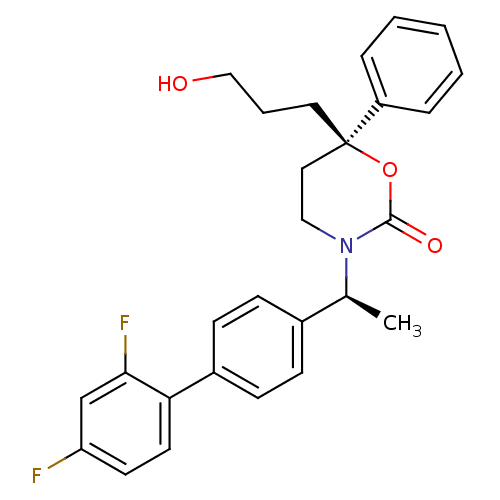 (CHEMBL1829759 | US8575157, 196 | US8592410, 93 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol after 1 hr by scintillation proximity... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353394 (CHEMBL1829767) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol after 1 hr by scintillation proximity... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353400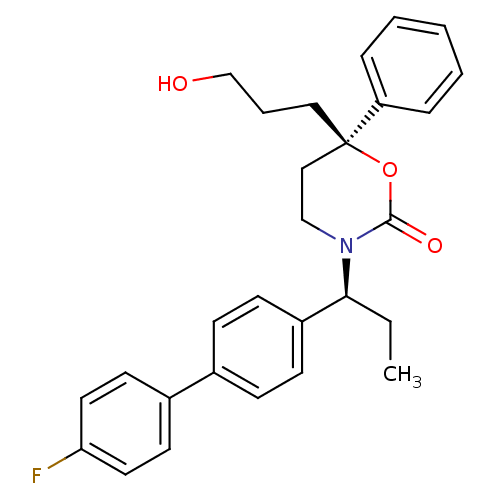 (CHEMBL1829762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol after 1 hr by scintillation proximity... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM195118 (US10336717, Compound 178 | US9212153, 178,Ex. 139) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2WH2NCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305462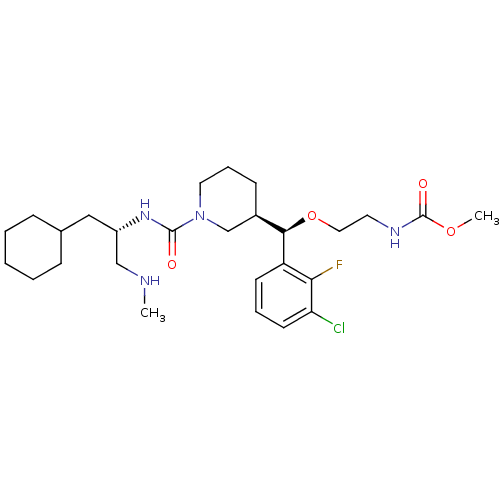 (CHEMBL590623 | methyl 2-((R)-(3-chloro-2-fluorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305463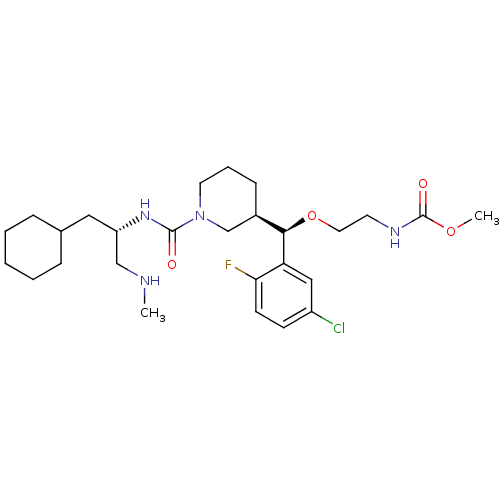 (CHEMBL590388 | methyl 2-((R)-(5-chloro-2-fluorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50305461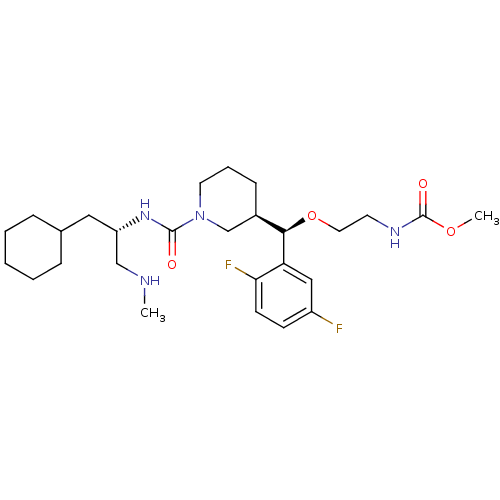 (CHEMBL606238 | methyl 2-((R)-((R)-1-((S)-1-cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of renin in human plasma assessed as formation of angiotensin1 product after 90 mins by competitive radioimmunoassay | ACS Med Chem Lett 2: 747-751 (2011) Article DOI: 10.1021/ml200137x BindingDB Entry DOI: 10.7270/Q2FT8N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human recombinant renin assessed as decrease in plasma renin activity by competitive radioimmunoassay in presence of human plasma | Bioorg Med Chem Lett 20: 694-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.066 BindingDB Entry DOI: 10.7270/Q24Q7V20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253507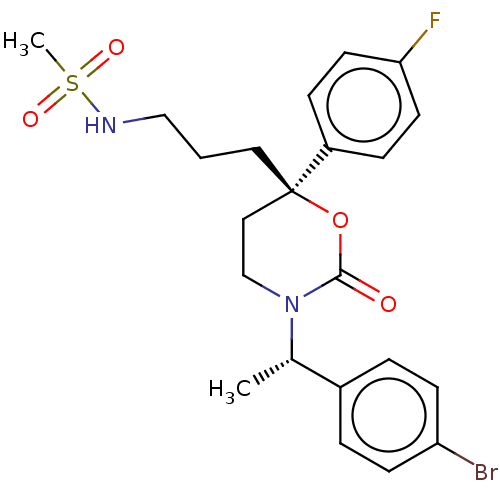 (CHEMBL4090672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1883 total ) | Next | Last >> |