

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173310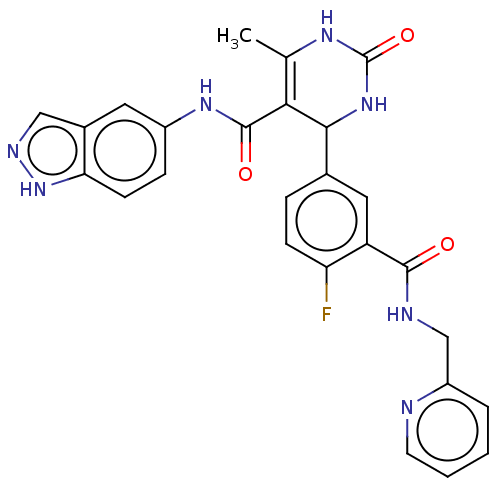 (CHEMBL3808660 | US10023564, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173307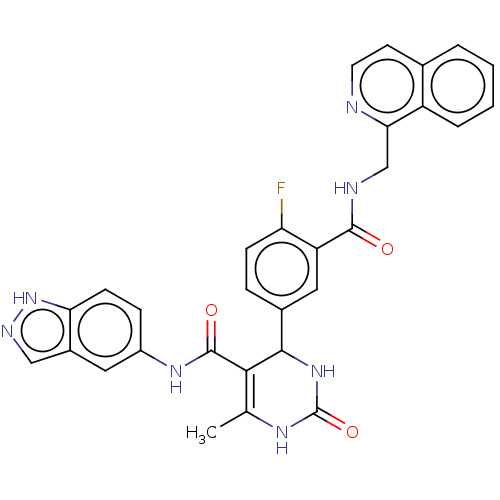 (CHEMBL3809796 | US10023564, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50173313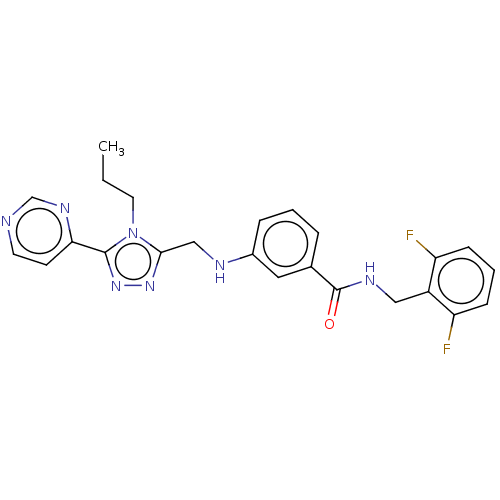 (CHEMBL1738878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50173313 (CHEMBL1738878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of GRK2 (unknown origin) | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173315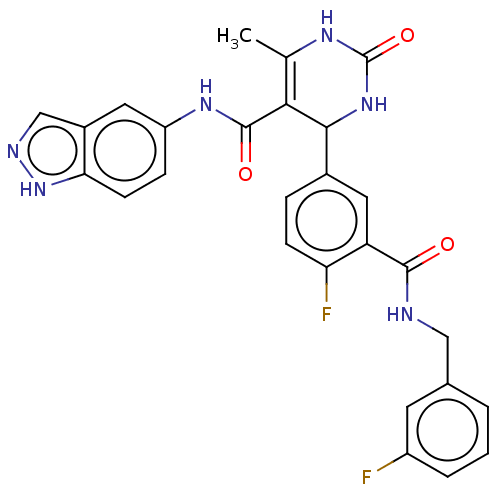 (CHEMBL3809100 | US10023564, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173305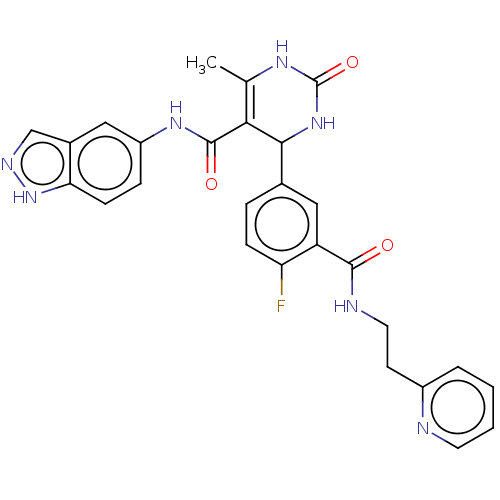 (CHEMBL3810107 | US10023564, Example 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50257350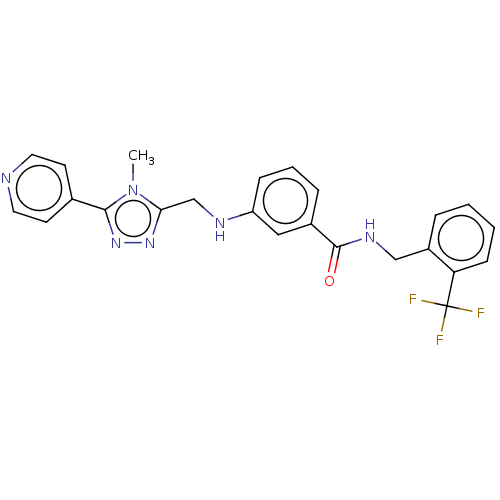 (CHEMBL1738877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260141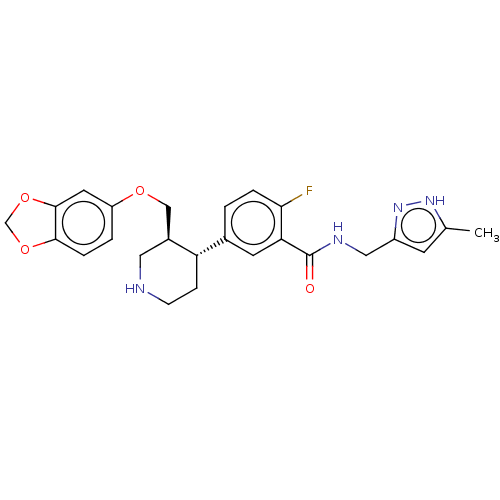 (CHEMBL4097393) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260140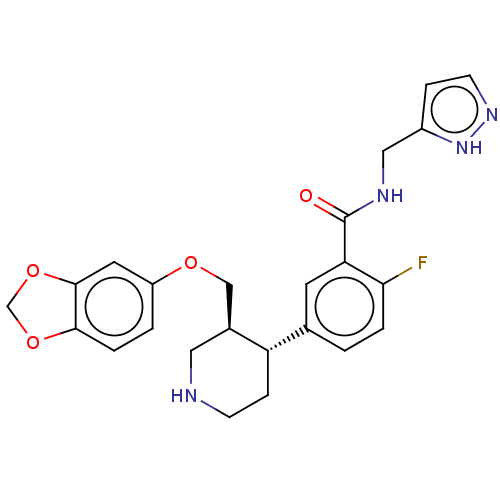 (CHEMBL4090144) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257350 (CHEMBL1738877) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of GRK2 (unknown origin) | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173311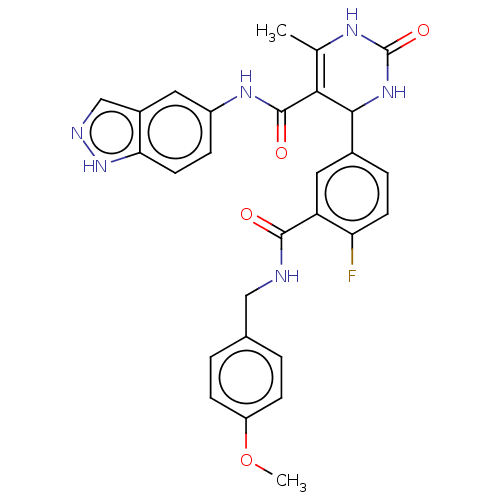 (CHEMBL3810073 | US10023564, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173325 (CHEMBL3809965 | US10023564, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173314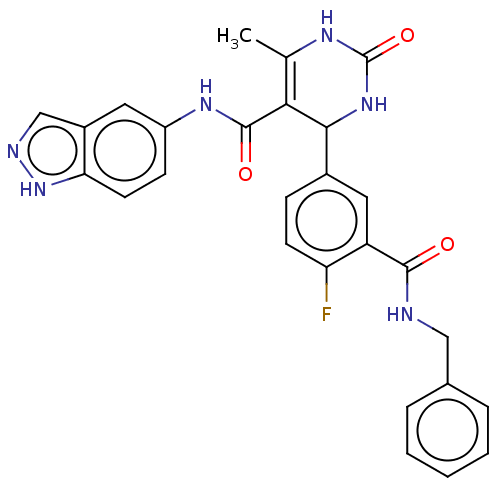 (CHEMBL3808840 | US10023564, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173306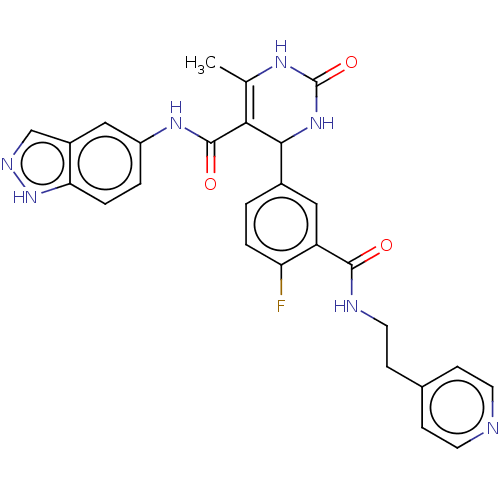 (CHEMBL3808565 | US10023564, Example 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173312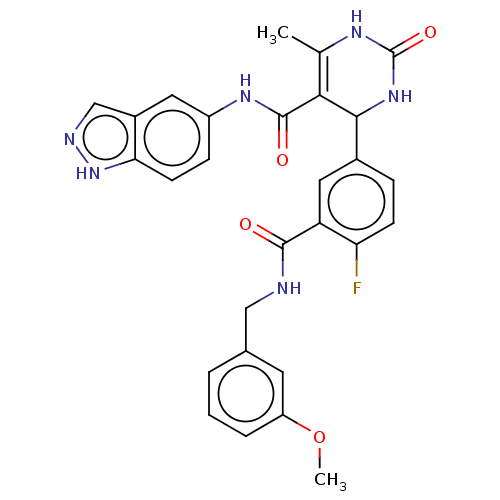 (CHEMBL3810312 | US10023564, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173307 (CHEMBL3809796 | US10023564, Example 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173320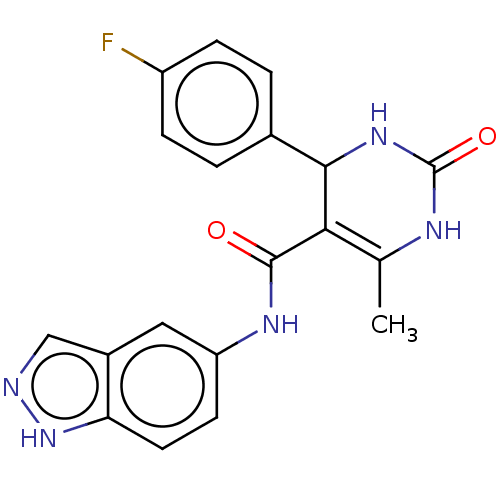 (GSK-180736A | GSK180736A | US10023564, Compound GS...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173323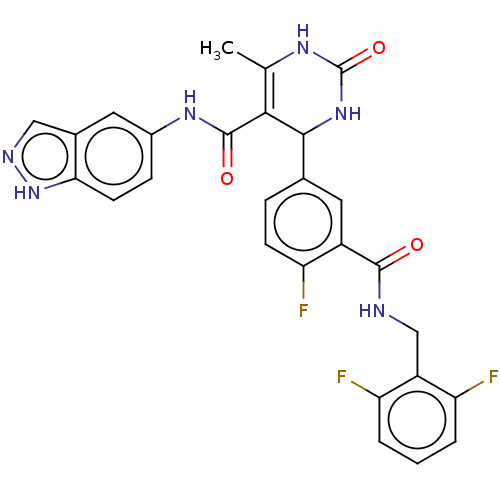 (CHEMBL3808621 | US10023564, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50173319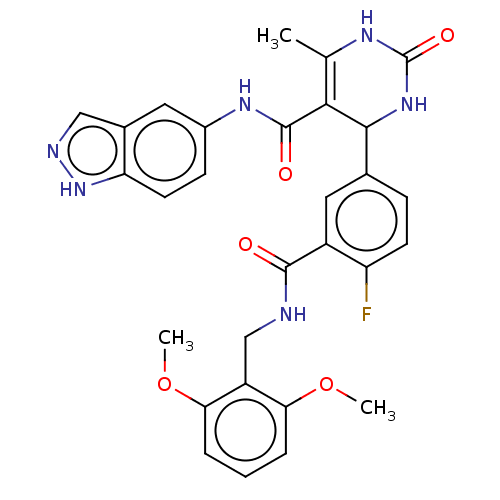 (CHEMBL3809020 | US10023564, Example 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of GRK2 (unknown origin) | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50173319 (CHEMBL3809020 | US10023564, Example 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173329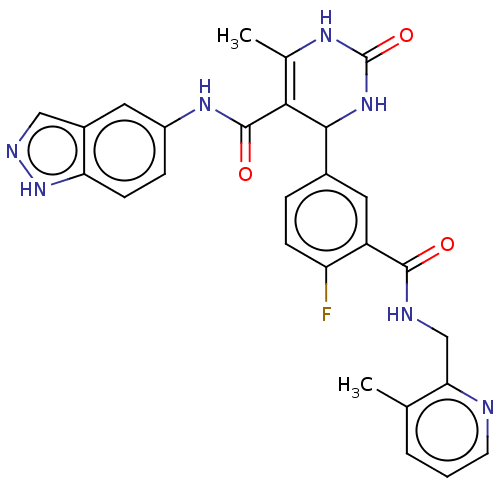 (CHEMBL3809697 | US10023564, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173316 (CHEMBL3810250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173324 (CHEMBL3809124 | US10023564, Example 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173328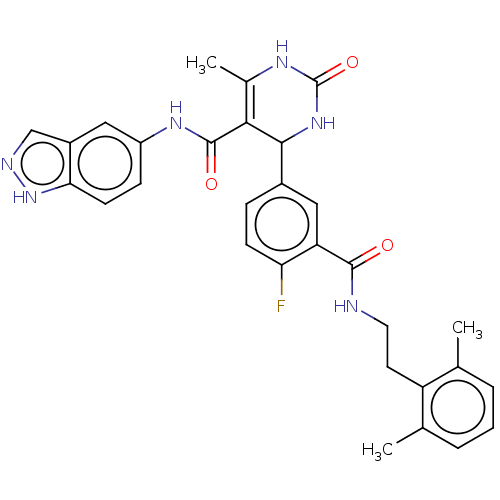 (CHEMBL3808918 | US10023564, Example 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50173310 (CHEMBL3808660 | US10023564, Example 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK5 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260143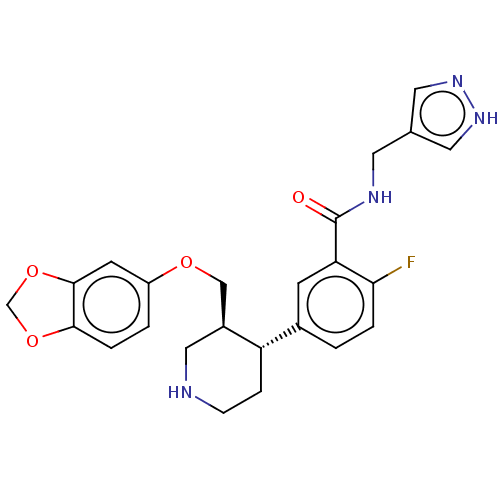 (CHEMBL4087244) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173326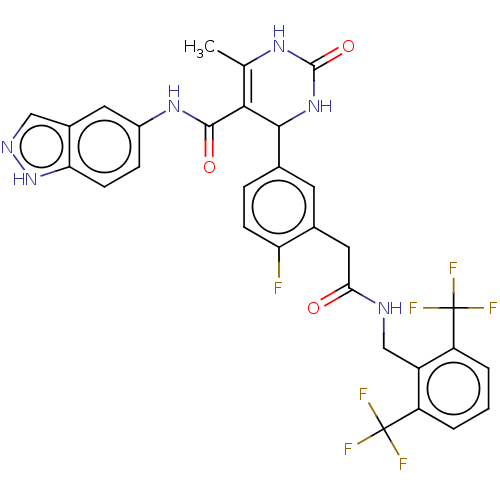 (CHEMBL3809604 | US10023564, Example 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173327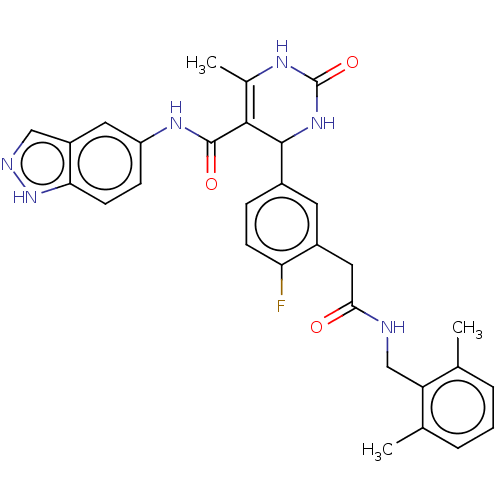 (CHEMBL3808499 | US10023564, Example 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173317 (CHEMBL3810349 | US10023564, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260139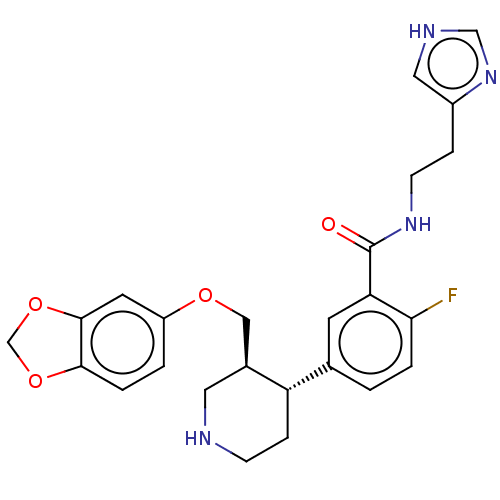 (CHEMBL4075712) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260148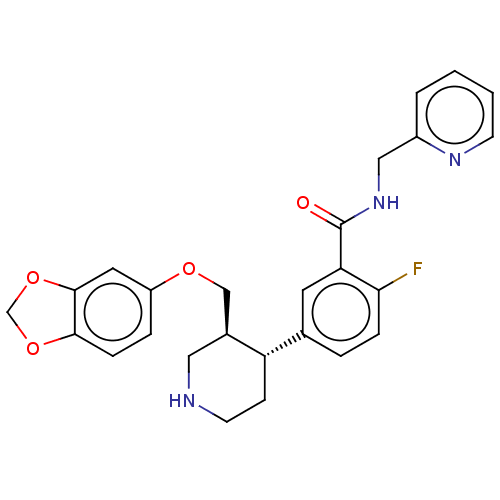 (CHEMBL4070885) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260145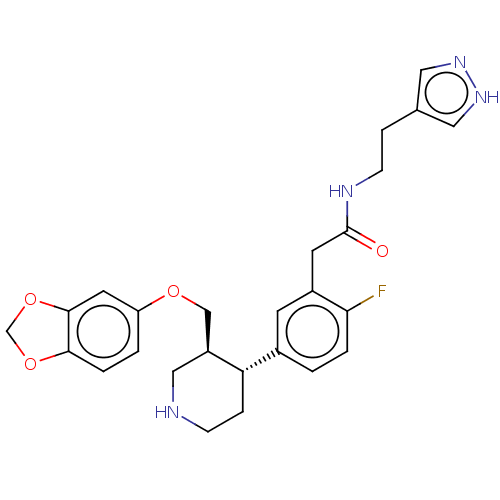 (CHEMBL4070290) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260138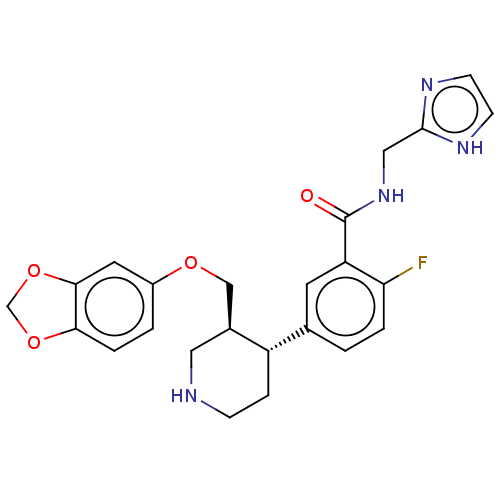 (CHEMBL4075475) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50173320 (GSK-180736A | GSK180736A | US10023564, Compound GS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of GRK2 (unknown origin) | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260125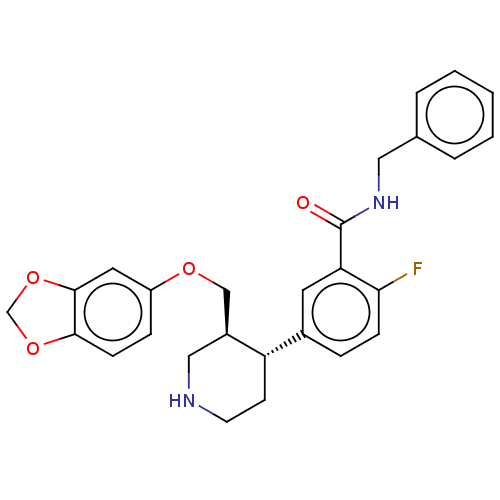 (CHEMBL4099398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50173320 (GSK-180736A | GSK180736A | US10023564, Compound GS...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260151 (CHEMBL4076529) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50173315 (CHEMBL3809100 | US10023564, Example 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK5 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260142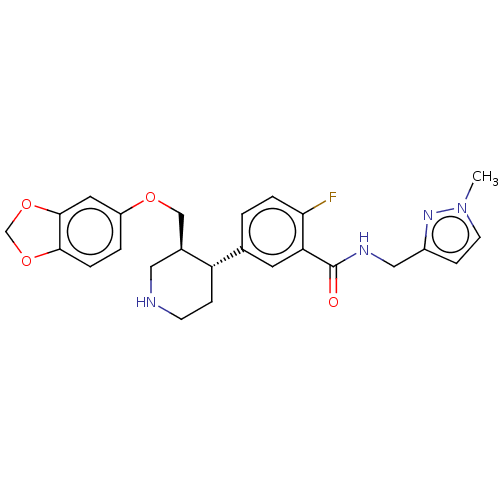 (CHEMBL4079362) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM22416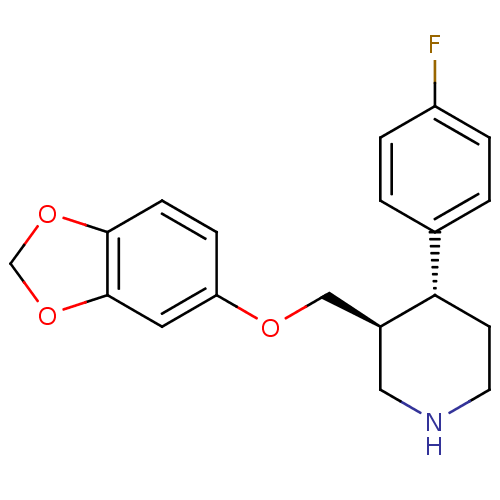 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity of compound towards dopamine transporter determined using [3H]WIN-35 428 as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260133 (CHEMBL4077197) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260127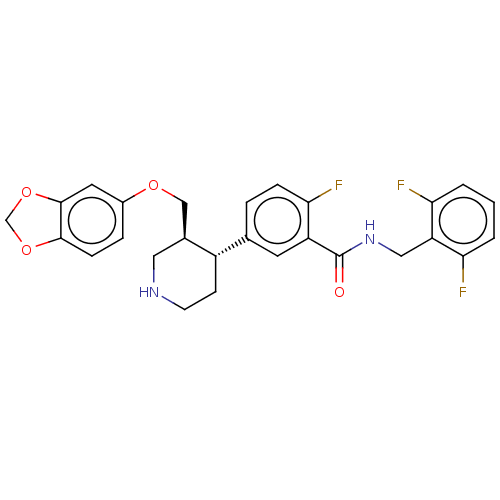 (CHEMBL4091281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260147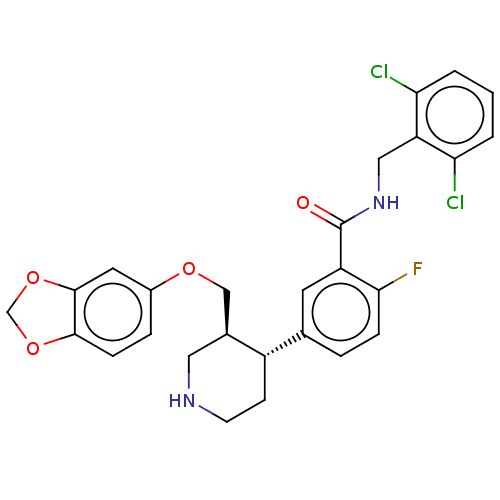 (CHEMBL4063014) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50173307 (CHEMBL3809796 | US10023564, Example 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK5 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173330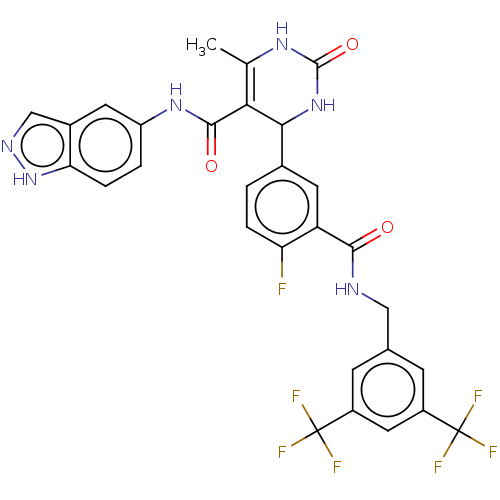 (CHEMBL3808657 | US10023564, Example 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260146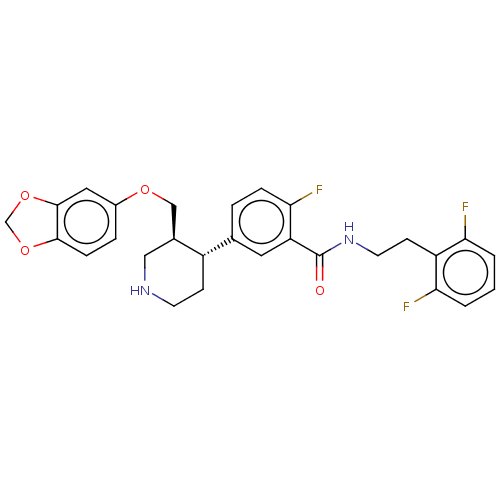 (CHEMBL4064274) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260134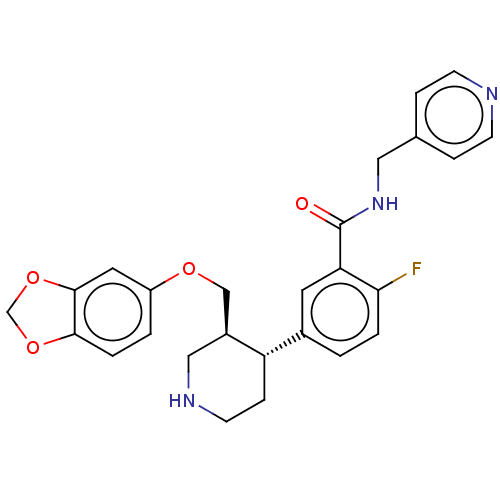 (CHEMBL4072898) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50260130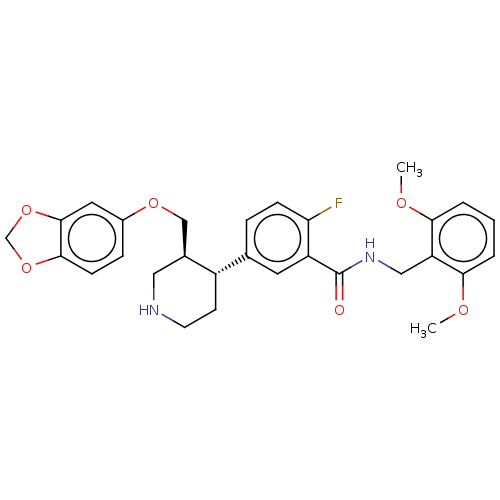 (CHEMBL4090923) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173322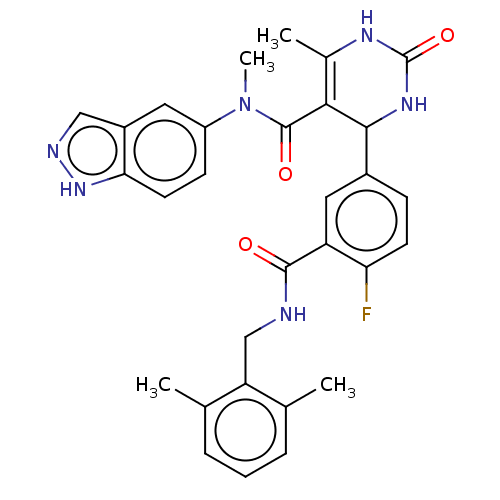 (CHEMBL3809584) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 183 total ) | Next | Last >> |