

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512575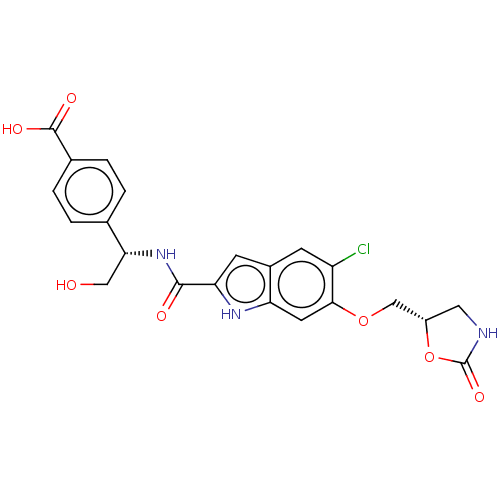 (CHEMBL4554985) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Mixed type inhibition of PHGDH (unknown origin) using 3-PG as substrate incubated for 20 mins in presence of , PSAT1, PSPH and varying NAD+ by diapho... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50230352 (CHEMBL4086943) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant human C-terminal His-tagged PHGDH (1 to 533 residues) expressed in Escherichia coli assessed as reduction i... | J Med Chem 60: 1591-1597 (2017) Article DOI: 10.1021/acs.jmedchem.6b01166 BindingDB Entry DOI: 10.7270/Q21N83CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50230352 (CHEMBL4086943) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant human C-terminal His-tagged PHGDH (1 to 533 residues) expressed in Escherichia coli assessed as reduction i... | J Med Chem 60: 1591-1597 (2017) Article DOI: 10.1021/acs.jmedchem.6b01166 BindingDB Entry DOI: 10.7270/Q21N83CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512584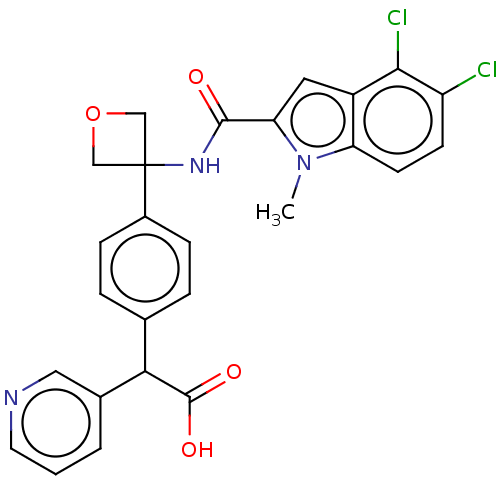 (CHEMBL4443265) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 11 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase ... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM15234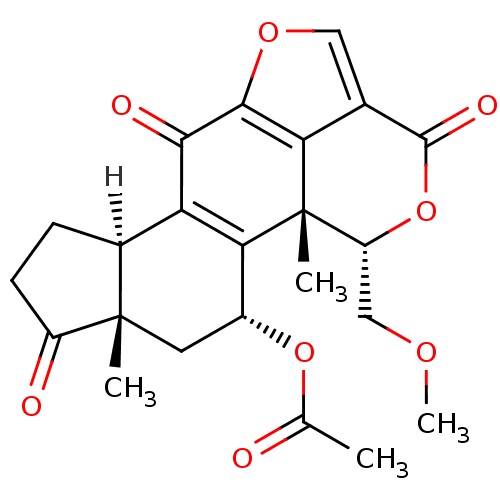 ((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262092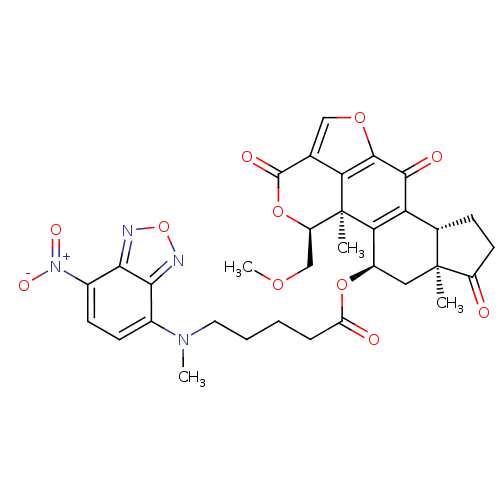 (5-[Methyl-(7-nitro-benzo[1,2,5]oxadiazol-4-yl)-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512573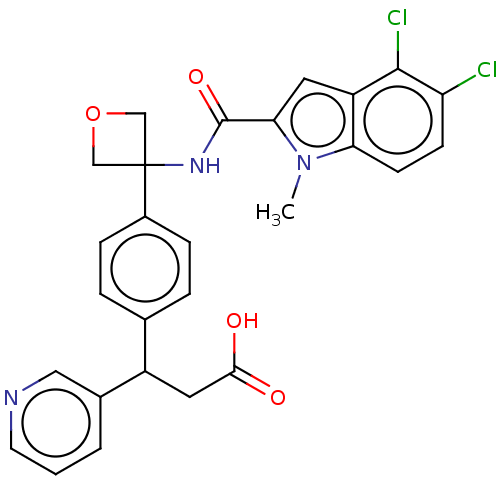 (CHEMBL4476654) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 11 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase ... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512582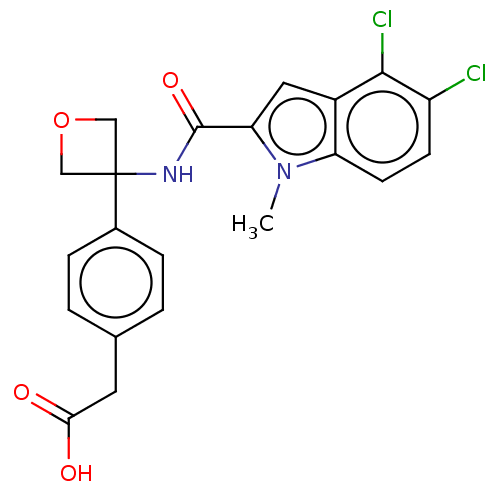 (CHEMBL4578644) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 11 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase ... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512587 (CHEMBL4452531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 11 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase ... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512589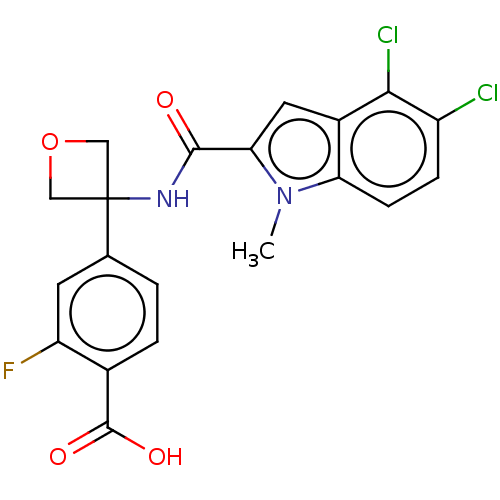 (CHEMBL4455598) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 11 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase ... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-phosphatidylinositol 3-phosphate 5-kinase (Homo sapiens) | BDBM50511422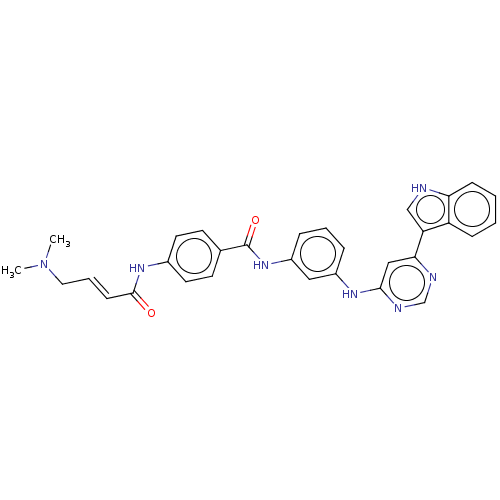 (CHEMBL4446338) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged full length human PIKFYVE (1 to 2098 residues) using PI(3)P and Phosphatidylserine as substrate by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262097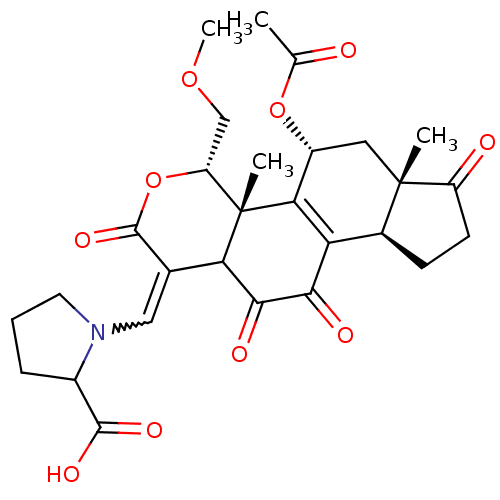 (1-((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a,6a-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262093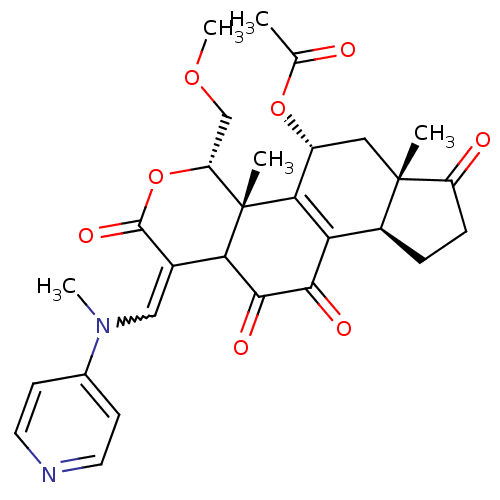 (11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-1-((me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262095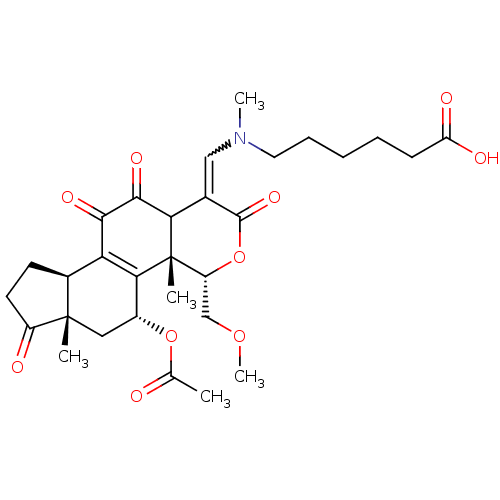 ((Z)-6-(((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50527943 (CHEMBL4447308) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant PI5P4Kalpha (unknown origin) incubated for 1 hr in presence of DPPS and PI5P by ADP-Glo assay | J Med Chem 63: 4880-4895 (2020) Article DOI: 10.1021/acs.jmedchem.0c00227 BindingDB Entry DOI: 10.7270/Q2SB4964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512574 (CHEMBL4471410) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262096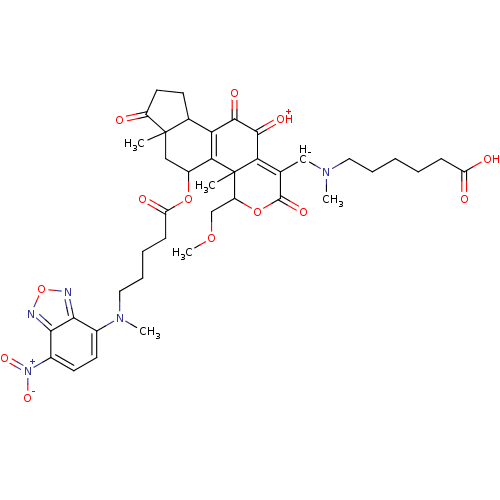 (6-(((11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512590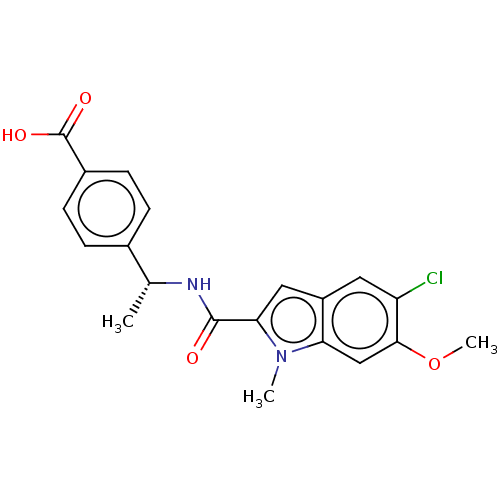 (CHEMBL4466146) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512571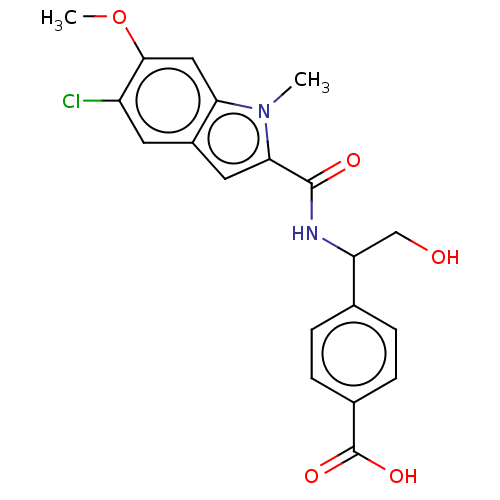 (CHEMBL4576611) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512575 (CHEMBL4554985) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) by surface plasmon resonance method | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512575 (CHEMBL4554985) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512575 (CHEMBL4554985) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262094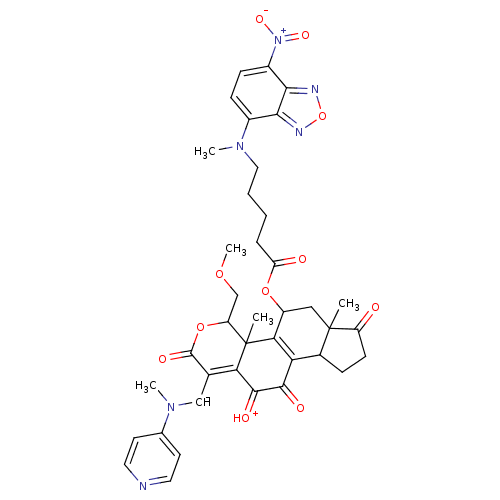 (11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-1-((me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50527927 (CHEMBL4436648) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant PI5P4Kalpha (unknown origin) incubated for 1 hr in presence of DPPS and PI5P by ADP-Glo assay | J Med Chem 63: 4880-4895 (2020) Article DOI: 10.1021/acs.jmedchem.0c00227 BindingDB Entry DOI: 10.7270/Q2SB4964 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512585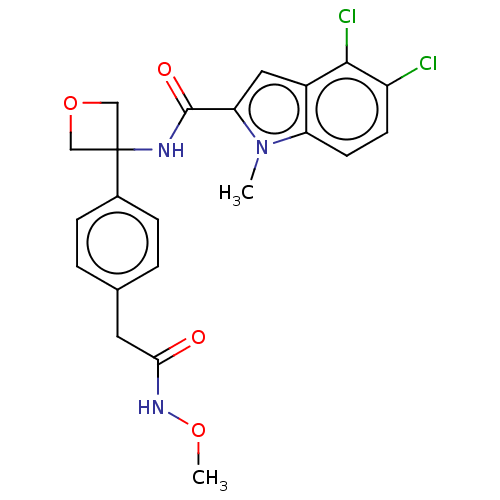 (CHEMBL4445276) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 287 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512580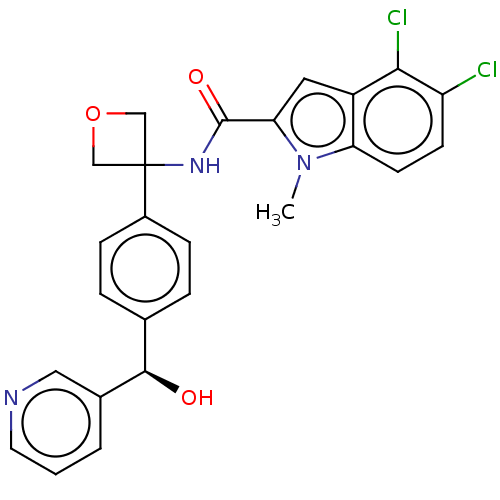 (CHEMBL4585294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512583 (CHEMBL4529734) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 11 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase ... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50527938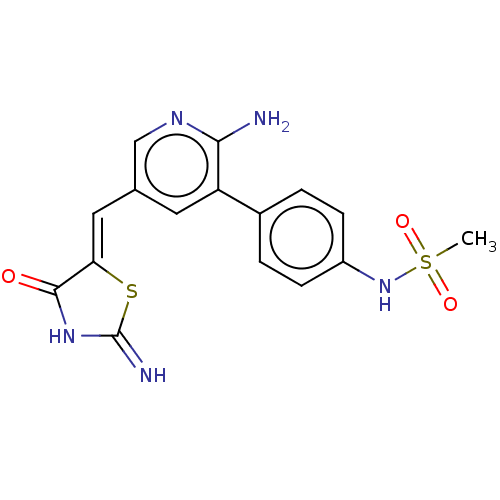 (CHEMBL4454033) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant PI5P4Kalpha (unknown origin) incubated for 1 hr in presence of DPPS and PI5P by ADP-Glo assay | J Med Chem 63: 4880-4895 (2020) Article DOI: 10.1021/acs.jmedchem.0c00227 BindingDB Entry DOI: 10.7270/Q2SB4964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511422 (CHEMBL4446338) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512572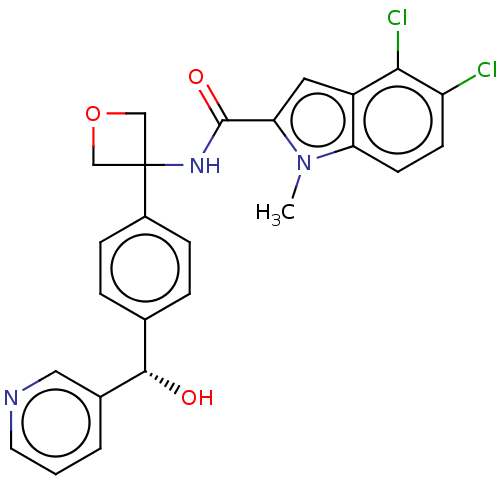 (CHEMBL4556384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511420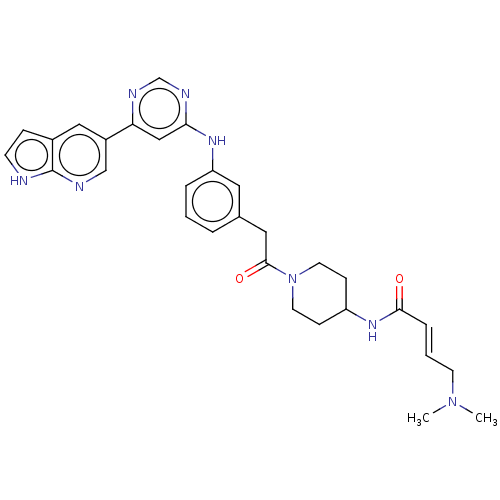 (CHEMBL4449493) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 gamma (Homo sapiens (Human)) | BDBM50497347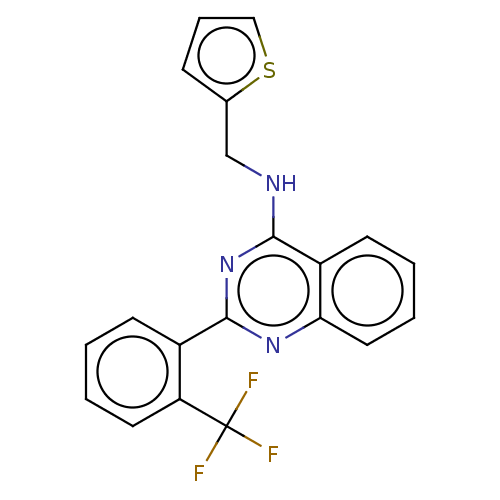 (CHEMBL1529478) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kgamma (unknown origin) | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM60983 ((E)-2-(3,4-dihydroxybenzoyl)-3-(4-hydroxy-3-iodo-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511414 (CHEMBL4472811) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511415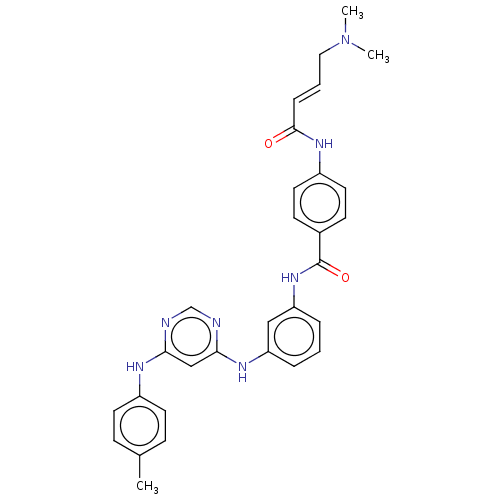 (CHEMBL4461694) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512581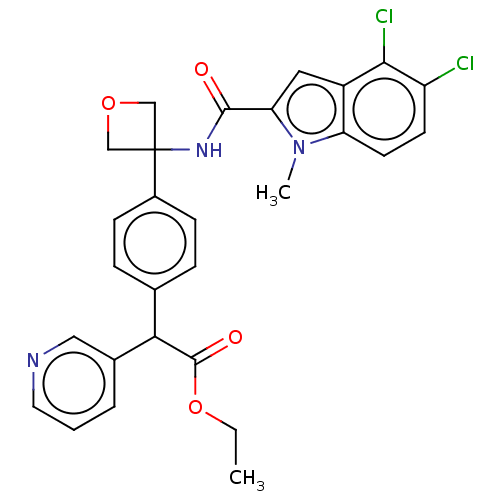 (CHEMBL4473812) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511421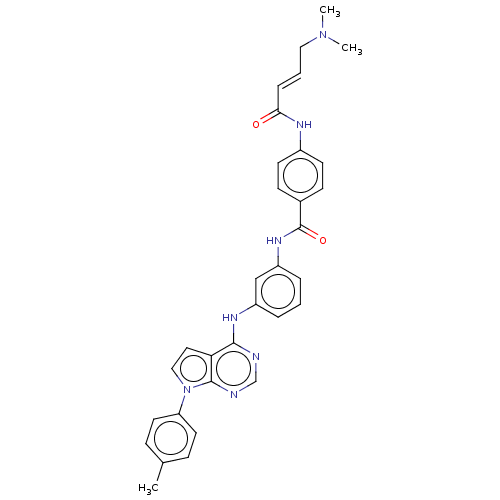 (CHEMBL4439287) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50527931 (CHEMBL4519752) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant PI5P4Kalpha (unknown origin) incubated for 1 hr in presence of DPPS and PI5P by ADP-Glo assay | J Med Chem 63: 4880-4895 (2020) Article DOI: 10.1021/acs.jmedchem.0c00227 BindingDB Entry DOI: 10.7270/Q2SB4964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512586 (CHEMBL4589459) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512579 (CHEMBL4442828) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 140 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 beta (Homo sapiens (Human)) | BDBM50527927 (CHEMBL4436648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant PI5P4Kbeta (unknown origin) incubated for 1 hr in presence of DPPS and PI5P by ADP-Glo assay | J Med Chem 63: 4880-4895 (2020) Article DOI: 10.1021/acs.jmedchem.0c00227 BindingDB Entry DOI: 10.7270/Q2SB4964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50527925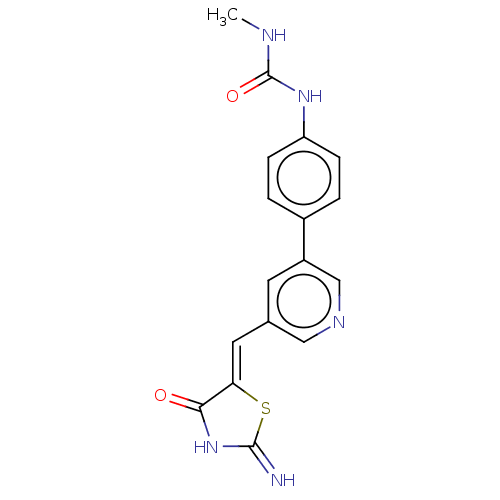 (CHEMBL4577337) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant PI5P4Kalpha (unknown origin) incubated for 1 hr in presence of DPPS and PI5P by ADP-Glo assay | J Med Chem 63: 4880-4895 (2020) Article DOI: 10.1021/acs.jmedchem.0c00227 BindingDB Entry DOI: 10.7270/Q2SB4964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 beta (Homo sapiens (Human)) | BDBM50527943 (CHEMBL4447308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant PI5P4Kbeta (unknown origin) incubated for 1 hr in presence of DPPS and PI5P by ADP-Glo assay | J Med Chem 63: 4880-4895 (2020) Article DOI: 10.1021/acs.jmedchem.0c00227 BindingDB Entry DOI: 10.7270/Q2SB4964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511410 (CHEMBL4284040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511425 (CHEMBL4572941) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50527929 (CHEMBL4455633) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant PI5P4Kalpha (unknown origin) incubated for 1 hr in presence of DPPS and PI5P by ADP-Glo assay | J Med Chem 63: 4880-4895 (2020) Article DOI: 10.1021/acs.jmedchem.0c00227 BindingDB Entry DOI: 10.7270/Q2SB4964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511419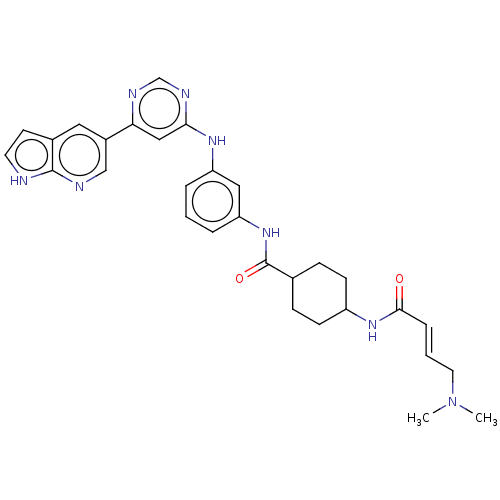 (CHEMBL4517979) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511413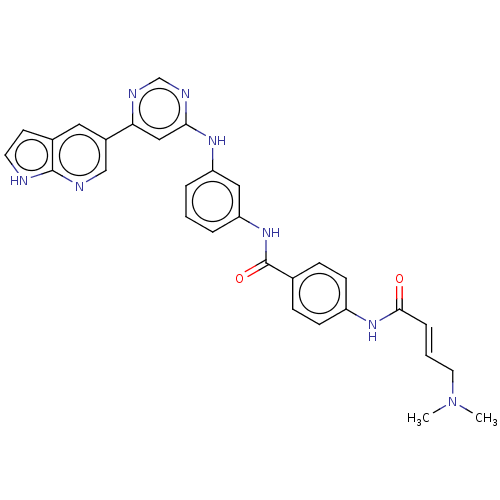 (CHEMBL4571099) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50511418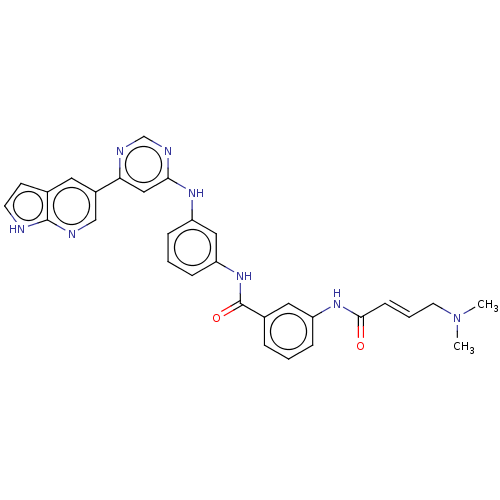 (CHEMBL4533123) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50527932 (CHEMBL4456203) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant PI5P4Kalpha (unknown origin) incubated for 1 hr in presence of DPPS and PI5P by ADP-Glo assay | J Med Chem 63: 4880-4895 (2020) Article DOI: 10.1021/acs.jmedchem.0c00227 BindingDB Entry DOI: 10.7270/Q2SB4964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 367 total ) | Next | Last >> |