

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysophosphatidic acid receptor 1/3 (Rattus norvegicus) | BDBM50496697 (CHEMBL3218460) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Antagonist activity at LPAR1/LPAR3 in rat glioma C62B cells assessed as inhibition of LPA-induced reduction in isoproterenol-stimulated [3H]cAMP accu... | Medchemcomm 2: 325-330 (2011) Article DOI: 10.1039/c0md00273a BindingDB Entry DOI: 10.7270/Q2FN1943 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50146232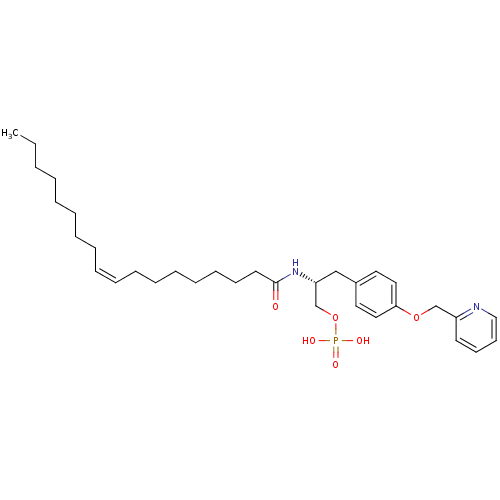 (CHEMBL440696 | Phosphoric acid mono-{(R)-2-((Z)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50150007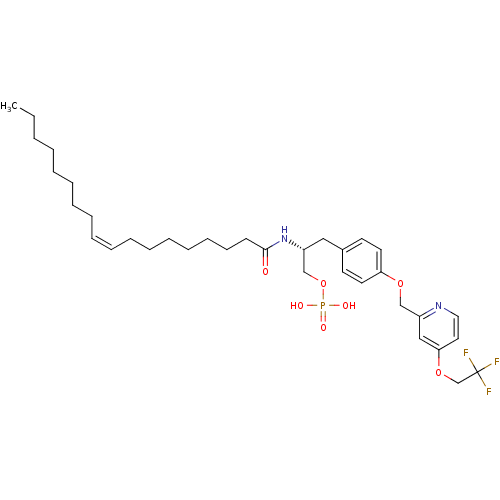 (CHEMBL183221 | Phosphoric acid mono-((R)-2-((Z)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50149996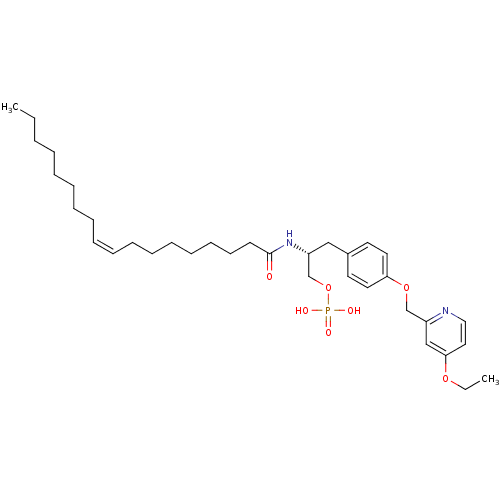 (CHEMBL183143 | Phosphoric acid mono-[3-[4-(4-ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50149997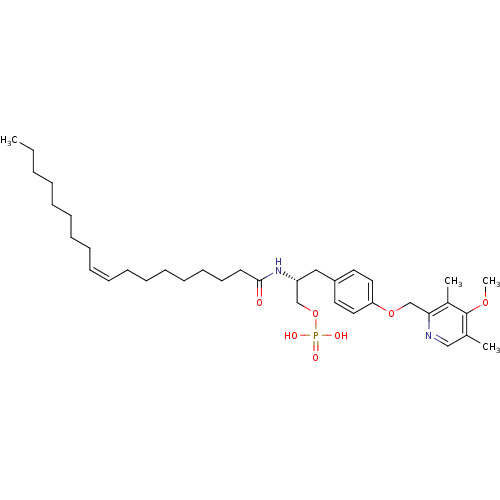 (CHEMBL362053 | Phosphoric acid mono-[3-[4-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50150000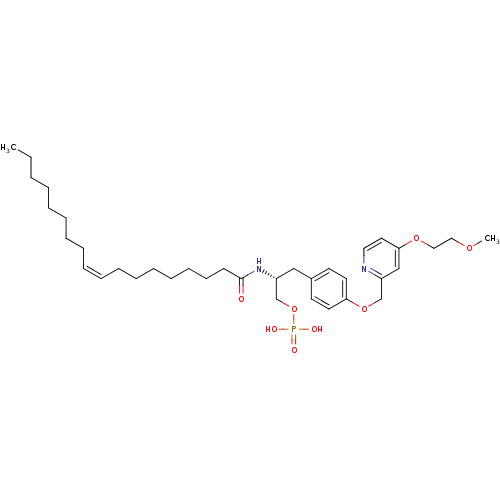 (CHEMBL182446 | Phosphoric acid mono-[(R)-3-{4-[4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50149996 (CHEMBL183143 | Phosphoric acid mono-[3-[4-(4-ethox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50149997 (CHEMBL362053 | Phosphoric acid mono-[3-[4-(4-metho...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50496698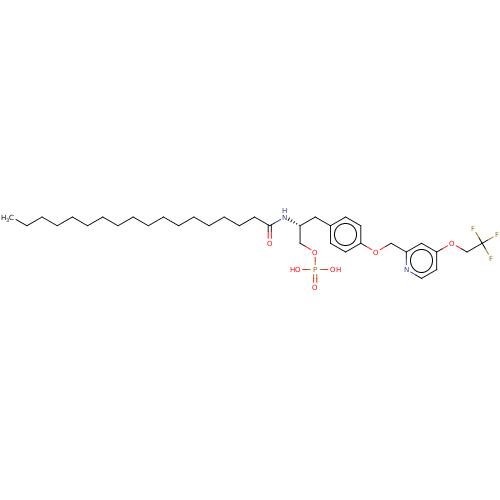 (CHEMBL3218459) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Competitive antagonist activity at N-terminal 3XHA-tagged human LPA3 receptor overexpressed in CHO cells assessed as inhibition of LPA-induced [35S]G... | Medchemcomm 2: 325-330 (2011) Article DOI: 10.1039/c0md00273a BindingDB Entry DOI: 10.7270/Q2FN1943 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150007 (CHEMBL183221 | Phosphoric acid mono-((R)-2-((Z)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50150014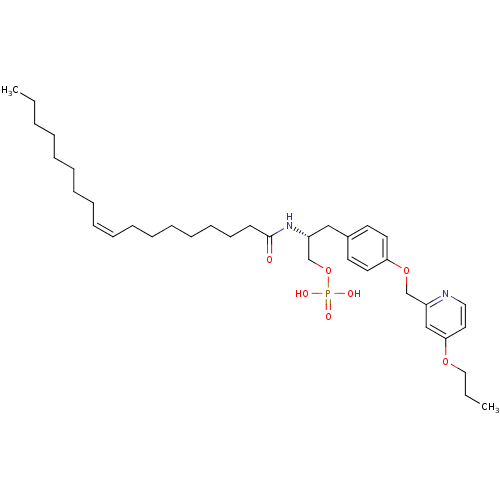 (CHEMBL291229 | Phosphoric acid mono-{2-((Z)-(R)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50496698 (CHEMBL3218459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Competitive antagonist activity at human LPA1 receptor overexpressed in CHO cells assessed as inhibition of LPA-induced [35S]GTPgammaS binding by liq... | Medchemcomm 2: 325-330 (2011) Article DOI: 10.1039/c0md00273a BindingDB Entry DOI: 10.7270/Q2FN1943 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150010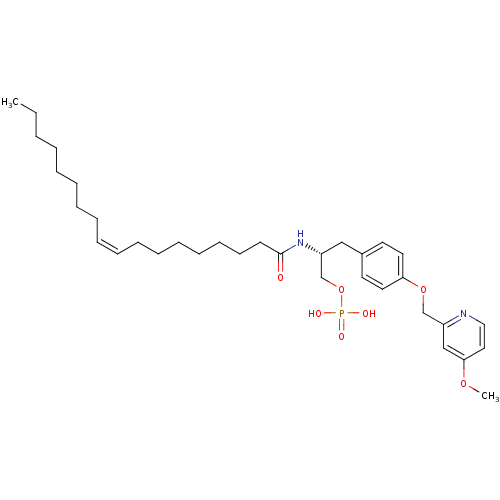 (CHEMBL265967 | Phosphoric acid mono-[(R)-3-[4-(4-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50496697 (CHEMBL3218460) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Competitive antagonist activity at human LPA1 receptor overexpressed in CHO cells assessed as inhibition of LPA-induced [35S]GTPgammaS binding by liq... | Medchemcomm 2: 325-330 (2011) Article DOI: 10.1039/c0md00273a BindingDB Entry DOI: 10.7270/Q2FN1943 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150008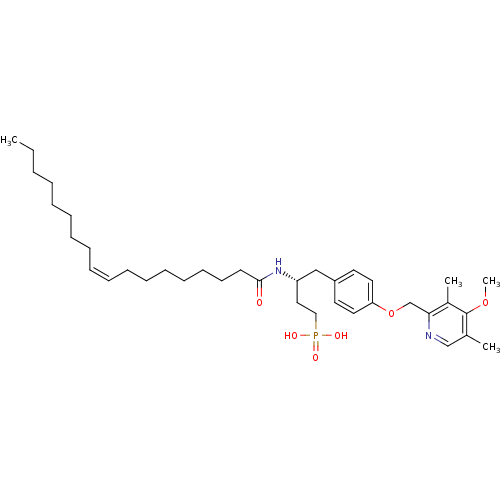 (CHEMBL182773 | [4-[4-(4-Methoxy-3,5-dimethyl-pyrid...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50146230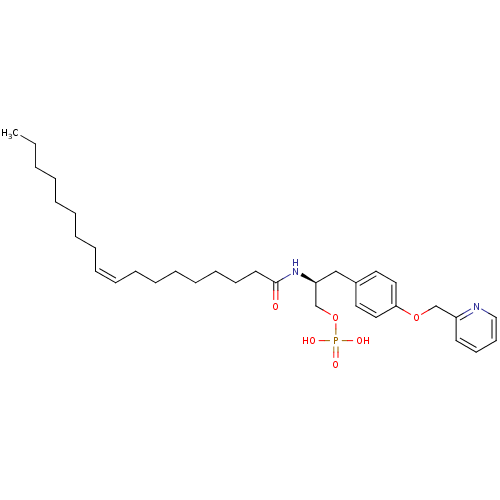 (CHEMBL314555 | Phosphoric acid mono-{(S)-2-((Z)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50146232 (CHEMBL440696 | Phosphoric acid mono-{(R)-2-((Z)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50149993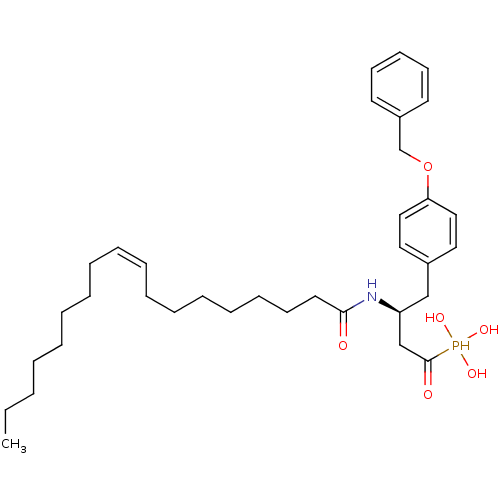 (CHEMBL181612 | [4-(4-Benzyloxy-phenyl)-1-hydroxy-3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150000 (CHEMBL182446 | Phosphoric acid mono-[(R)-3-{4-[4-(...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 423 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50149998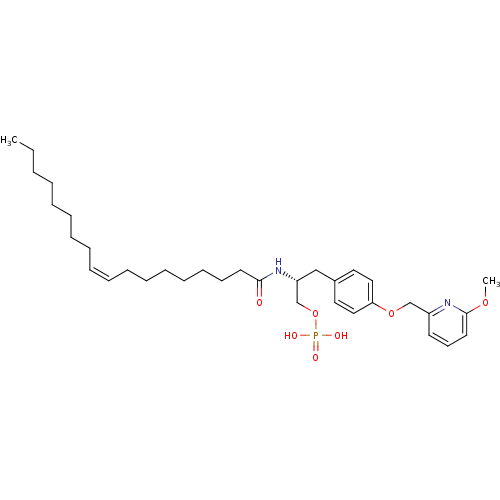 (CHEMBL185287 | Phosphoric acid mono-[(R)-3-[4-(6-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50496697 (CHEMBL3218460) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 512 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Competitive antagonist activity at N-terminal 3XHA-tagged human LPA3 receptor overexpressed in CHO cells assessed as inhibition of LPA-induced [35S]G... | Medchemcomm 2: 325-330 (2011) Article DOI: 10.1039/c0md00273a BindingDB Entry DOI: 10.7270/Q2FN1943 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150001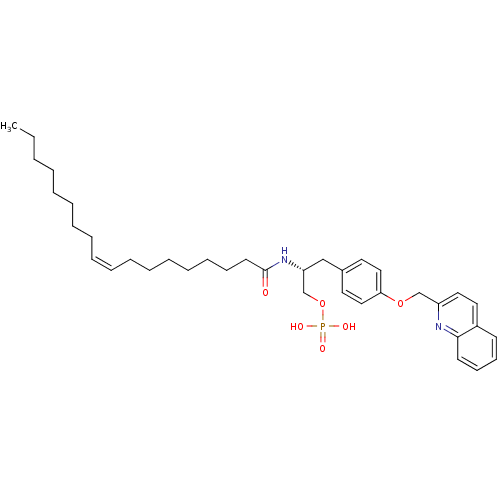 (CHEMBL360131 | Phosphoric acid mono-{2-((Z)-(R)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 533 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150009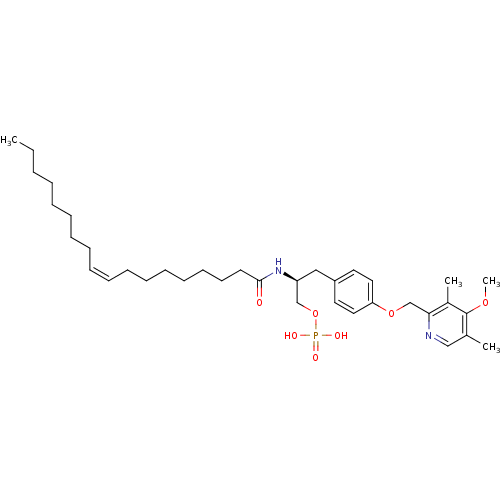 (CHEMBL181917 | Phosphoric acid mono-[(S)-3-[4-(4-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 561 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50146230 (CHEMBL314555 | Phosphoric acid mono-{(S)-2-((Z)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150004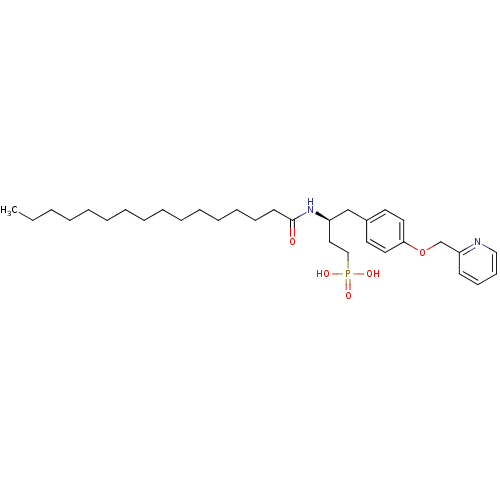 (CHEMBL360064 | {(S)-3-Hexadecanoylamino-4-[4-(pyri...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150005 (CHEMBL83782 | Phosphoric acid mono-[3-[4-(6-methox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150003 (CHEMBL360928 | Phosphoric acid mono-[(R)-3-[4-(4-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150014 (CHEMBL291229 | Phosphoric acid mono-{2-((Z)-(R)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150002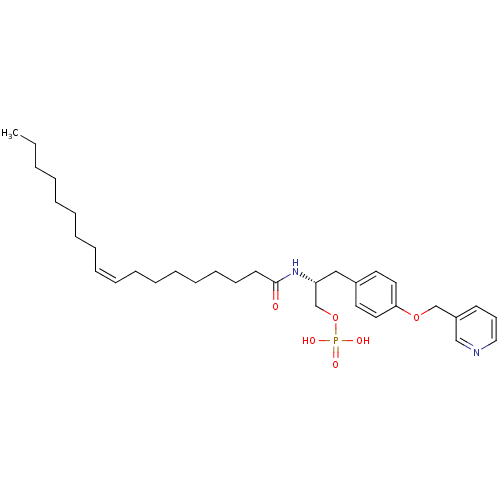 (CHEMBL184055 | Phosphoric acid mono-{(R)-2-((Z)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50149995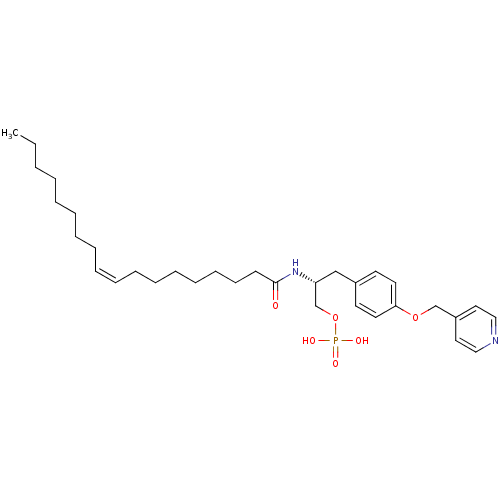 (CHEMBL360399 | Phosphoric acid mono-{(R)-2-((Z)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150011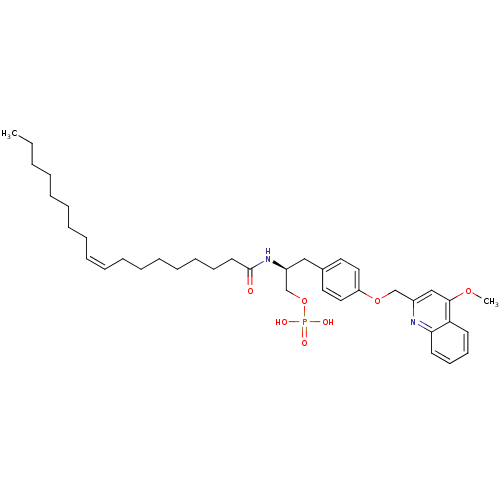 (CHEMBL182302 | Phosphoric acid mono-[3-[4-(4-metho...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150006 (CHEMBL366292 | [(S)-4-[4-(4-Methoxy-3,5-dimethyl-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50149994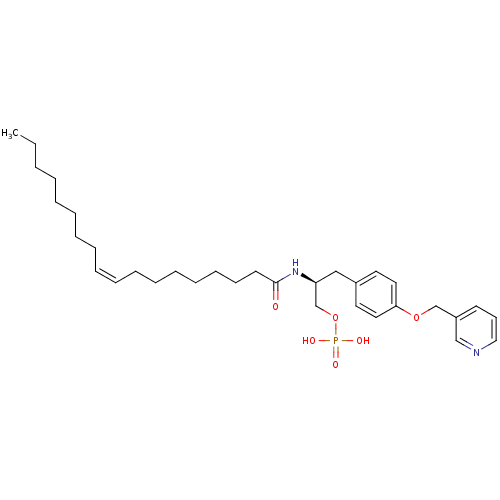 (CHEMBL360727 | Phosphoric acid mono-{2-((Z)-(S)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50149999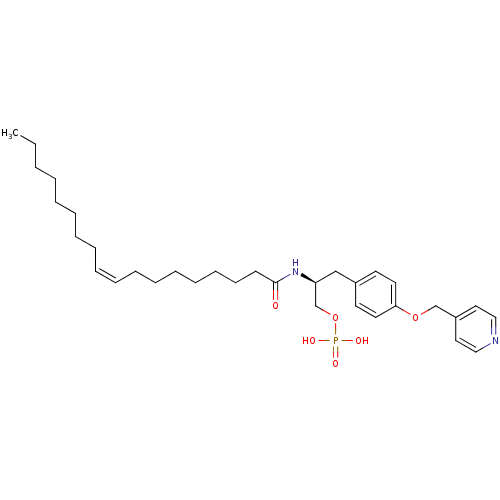 (CHEMBL360397 | Phosphoric acid mono-{2-((Z)-(S)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150012 (CHEMBL181906 | {3-((Z)-(S)-Octadec-9-enoylamino)-4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150013 (CHEMBL181956 | {(R)-3-((Z)-Octadec-9-enoylamino)-4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM3152 (2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3153 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3152 (2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3152 (2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3153 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3153 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3235 ((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3230 ((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM3153 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3152 (2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50149997 (CHEMBL362053 | Phosphoric acid mono-[3-[4-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibitory concentration against Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 288 total ) | Next | Last >> |