 Found 65 hits with Last Name = 'caulkett' and Initial = 'pw'
Found 65 hits with Last Name = 'caulkett' and Initial = 'pw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50006730
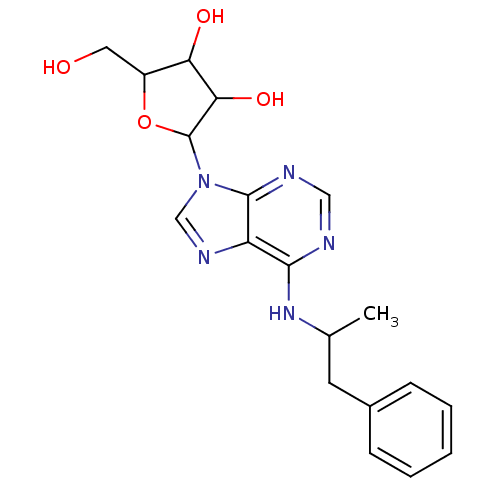
((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM35804

((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(CCC(O)=O)cc3)nc12 Show InChI InChI=1S/C23H29N7O6/c1-2-25-21(35)18-16(33)17(34)22(36-18)30-11-27-15-19(24)28-23(29-20(15)30)26-10-9-13-5-3-12(4-6-13)7-8-14(31)32/h3-6,11,16-18,22,33-34H,2,7-10H2,1H3,(H,25,35)(H,31,32)(H3,24,26,28,29)/t16-,17+,18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 335 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50003588
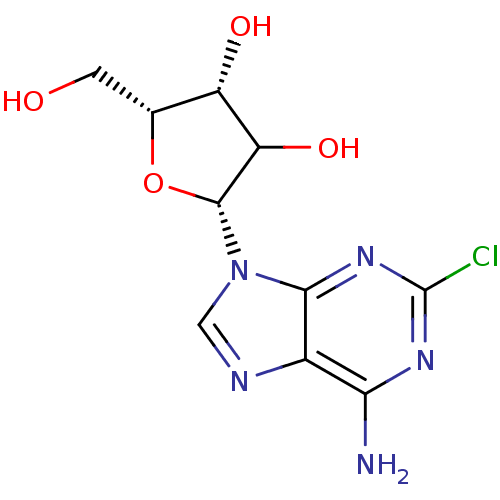
((2-CADO) 2-(6-Amino-2-chloro-purin-9-yl)-5-hydroxy...)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@H](O)C1O Show InChI InChI=1S/C10H12ClN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5+,6?,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 913 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50006730

((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 989 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM35804

((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(CCC(O)=O)cc3)nc12 Show InChI InChI=1S/C23H29N7O6/c1-2-25-21(35)18-16(33)17(34)22(36-18)30-11-27-15-19(24)28-23(29-20(15)30)26-10-9-13-5-3-12(4-6-13)7-8-14(31)32/h3-6,11,16-18,22,33-34H,2,7-10H2,1H3,(H,25,35)(H,31,32)(H3,24,26,28,29)/t16-,17+,18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM82023
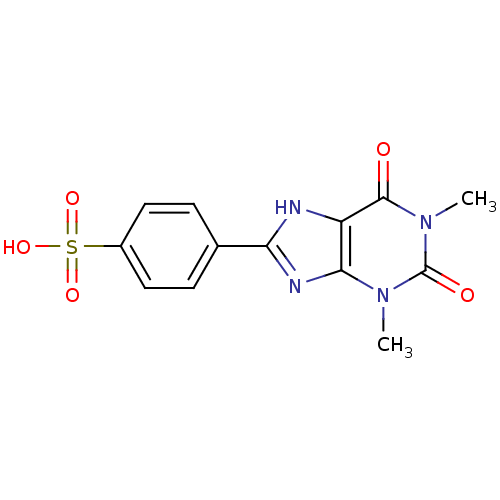
(4-(1,3-Dimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-pu...)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(cc1)S(O)(=O)=O Show InChI InChI=1S/C13H12N4O5S/c1-16-11-9(12(18)17(2)13(16)19)14-10(15-11)7-3-5-8(6-4-7)23(20,21)22/h3-6H,1-2H3,(H,14,15)(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM82015
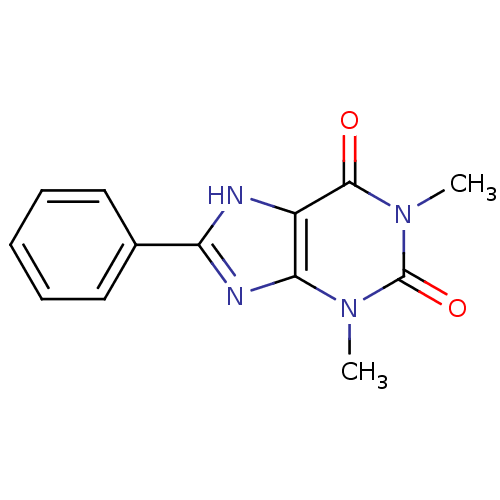
(1,3-Dimethyl-8-phenyl-3,9-dihydro-purine-2,6-dione...)Show InChI InChI=1S/C13H12N4O2/c1-16-11-9(12(18)17(2)13(16)19)14-10(15-11)8-6-4-3-5-7-8/h3-7H,1-2H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165030
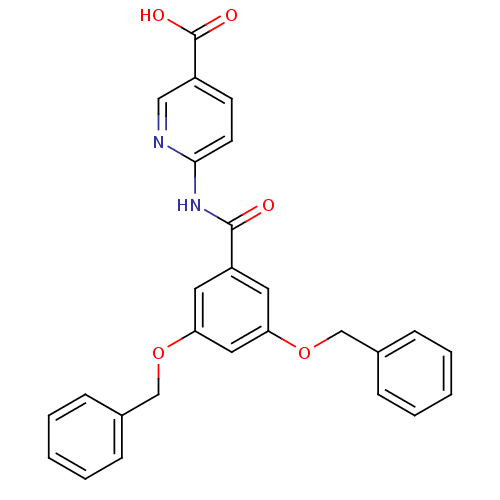
(6-(3,5-Bis-benzyloxy-benzoylamino)-nicotinic acid ...)Show SMILES OC(=O)c1ccc(NC(=O)c2cc(OCc3ccccc3)cc(OCc3ccccc3)c2)nc1 Show InChI InChI=1S/C27H22N2O5/c30-26(29-25-12-11-21(16-28-25)27(31)32)22-13-23(33-17-19-7-3-1-4-8-19)15-24(14-22)34-18-20-9-5-2-6-10-20/h1-16H,17-18H2,(H,31,32)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165031
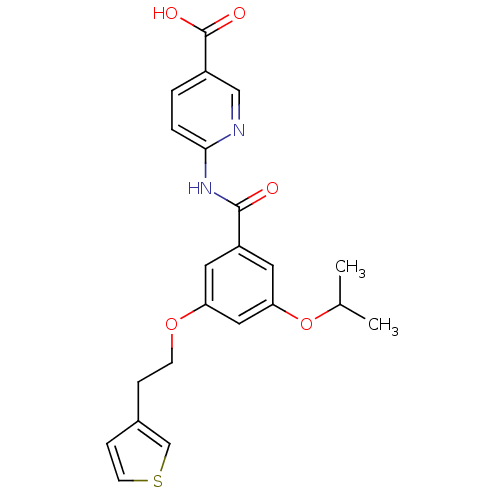
(6-({3-isopropoxy-5-[2-(3-thienyl)ethoxy]benzoyl}am...)Show SMILES CC(C)Oc1cc(OCCc2ccsc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C22H22N2O5S/c1-14(2)29-19-10-17(9-18(11-19)28-7-5-15-6-8-30-13-15)21(25)24-20-4-3-16(12-23-20)22(26)27/h3-4,6,8-14H,5,7H2,1-2H3,(H,26,27)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165032

(6-(2-Benzyloxy-5-methylsulfanyl-phenylcarbamoyl)-n...)Show SMILES CSc1ccc(OCc2ccccc2)c(NC(=O)c2ccc(cn2)C(O)=O)c1 Show InChI InChI=1S/C21H18N2O4S/c1-28-16-8-10-19(27-13-14-5-3-2-4-6-14)18(11-16)23-20(24)17-9-7-15(12-22-17)21(25)26/h2-12H,13H2,1H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165034

(6-[3-Isopropoxy-5-(2-pyridin-4-yl-ethoxy)-benzoyla...)Show SMILES CC(C)Oc1cc(OCCc2ccncc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C23H23N3O5/c1-15(2)31-20-12-18(22(27)26-21-4-3-17(14-25-21)23(28)29)11-19(13-20)30-10-7-16-5-8-24-9-6-16/h3-6,8-9,11-15H,7,10H2,1-2H3,(H,28,29)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165033

(6-[2,3-Bis-(2-chloro-benzyloxy)-benzoylamino]-nico...)Show SMILES OC(=O)c1ccc(NC(=O)c2cccc(OCc3ccccc3Cl)c2OCc2ccccc2Cl)nc1 Show InChI InChI=1S/C27H20Cl2N2O5/c28-21-9-3-1-6-18(21)15-35-23-11-5-8-20(25(23)36-16-19-7-2-4-10-22(19)29)26(32)31-24-13-12-17(14-30-24)27(33)34/h1-14H,15-16H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165035
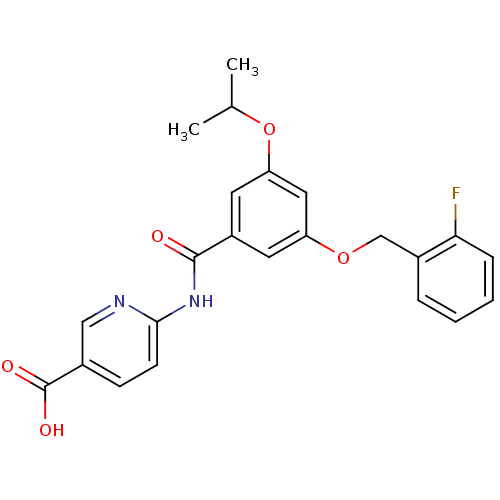
(6-[3-(2-Fluoro-benzyloxy)-5-isopropoxy-benzoylamin...)Show SMILES CC(C)Oc1cc(OCc2ccccc2F)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C23H21FN2O5/c1-14(2)31-19-10-17(22(27)26-21-8-7-15(12-25-21)23(28)29)9-18(11-19)30-13-16-5-3-4-6-20(16)24/h3-12,14H,13H2,1-2H3,(H,28,29)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165037

(6-(3-Isobutoxy-5-isopropoxy-benzoylamino)-nicotini...)Show SMILES CC(C)COc1cc(OC(C)C)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C20H24N2O5/c1-12(2)11-26-16-7-15(8-17(9-16)27-13(3)4)19(23)22-18-6-5-14(10-21-18)20(24)25/h5-10,12-13H,11H2,1-4H3,(H,24,25)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165036
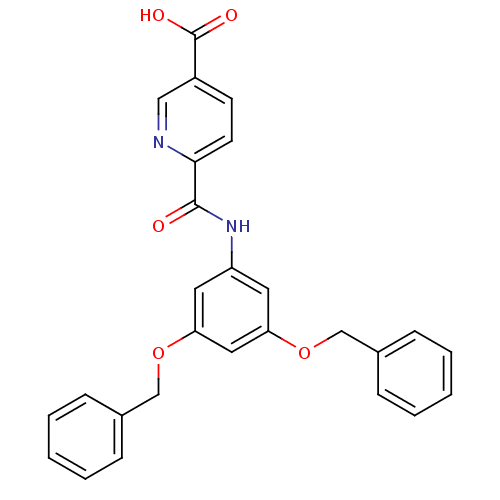
(6-(3,5-Bis-benzyloxy-phenylcarbamoyl)-nicotinic ac...)Show SMILES OC(=O)c1ccc(nc1)C(=O)Nc1cc(OCc2ccccc2)cc(OCc2ccccc2)c1 Show InChI InChI=1S/C27H22N2O5/c30-26(25-12-11-21(16-28-25)27(31)32)29-22-13-23(33-17-19-7-3-1-4-8-19)15-24(14-22)34-18-20-9-5-2-6-10-20/h1-16H,17-18H2,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165038

(6-(3,5-Bis-benzyloxy-benzyloxy)-nicotinic acid | C...)Show SMILES OC(=O)c1ccc(OCc2cc(OCc3ccccc3)cc(OCc3ccccc3)c2)nc1 Show InChI InChI=1S/C27H23NO5/c29-27(30)23-11-12-26(28-16-23)33-19-22-13-24(31-17-20-7-3-1-4-8-20)15-25(14-22)32-18-21-9-5-2-6-10-21/h1-16H,17-19H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165040

(6-(3-Isopropoxy-5-phenethyloxy-benzoylamino)-nicot...)Show SMILES CC(C)Oc1cc(OCCc2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C24H24N2O5/c1-16(2)31-21-13-19(23(27)26-22-9-8-18(15-25-22)24(28)29)12-20(14-21)30-11-10-17-6-4-3-5-7-17/h3-9,12-16H,10-11H2,1-2H3,(H,28,29)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165039

(6-[3-(2-Chloro-benzyloxy)-benzoylamino]-nicotinic ...)Show SMILES OC(=O)c1ccc(NC(=O)c2cccc(OCc3ccccc3Cl)c2)nc1 Show InChI InChI=1S/C20H15ClN2O4/c21-17-7-2-1-4-15(17)12-27-16-6-3-5-13(10-16)19(24)23-18-9-8-14(11-22-18)20(25)26/h1-11H,12H2,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 910 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165041

(6-(3-(benzyloxy)-5-isopropoxybenzamido)nicotinic a...)Show SMILES CC(C)Oc1cc(OCc2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C23H22N2O5/c1-15(2)30-20-11-18(10-19(12-20)29-14-16-6-4-3-5-7-16)22(26)25-21-9-8-17(13-24-21)23(27)28/h3-13,15H,14H2,1-2H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165042

(6-(3,5-Dimethoxy-phenoxymethyl)-nicotinic acid | C...)Show InChI InChI=1S/C15H15NO5/c1-19-12-5-13(20-2)7-14(6-12)21-9-11-4-3-10(8-16-11)15(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165044

(6-(3-Cyclopentylmethoxy-5-isopropoxy-benzoylamino)...)Show SMILES CC(C)Oc1cc(OCC2CCCC2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C22H26N2O5/c1-14(2)29-19-10-17(9-18(11-19)28-13-15-5-3-4-6-15)21(25)24-20-8-7-16(12-23-20)22(26)27/h7-12,14-15H,3-6,13H2,1-2H3,(H,26,27)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 650 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165043
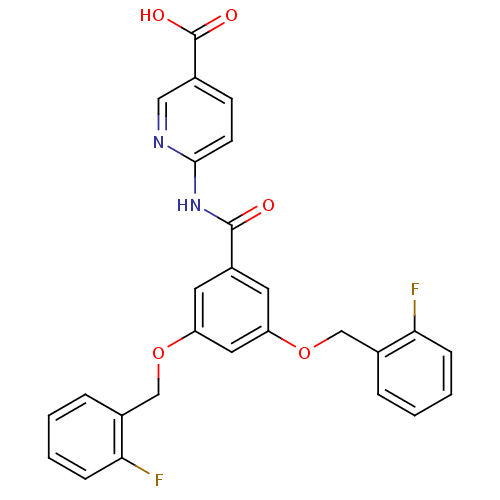
(6-[3,5-Bis-(2-fluoro-benzyloxy)-benzoylamino]-nico...)Show SMILES OC(=O)c1ccc(NC(=O)c2cc(OCc3ccccc3F)cc(OCc3ccccc3F)c2)nc1 Show InChI InChI=1S/C27H20F2N2O5/c28-23-7-3-1-5-18(23)15-35-21-11-20(26(32)31-25-10-9-17(14-30-25)27(33)34)12-22(13-21)36-16-19-6-2-4-8-24(19)29/h1-14H,15-16H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165046

(6-(2-Benzyloxy-benzoylamino)-nicotinic acid | CHEM...)Show InChI InChI=1S/C20H16N2O4/c23-19(22-18-11-10-15(12-21-18)20(24)25)16-8-4-5-9-17(16)26-13-14-6-2-1-3-7-14/h1-12H,13H2,(H,24,25)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165047
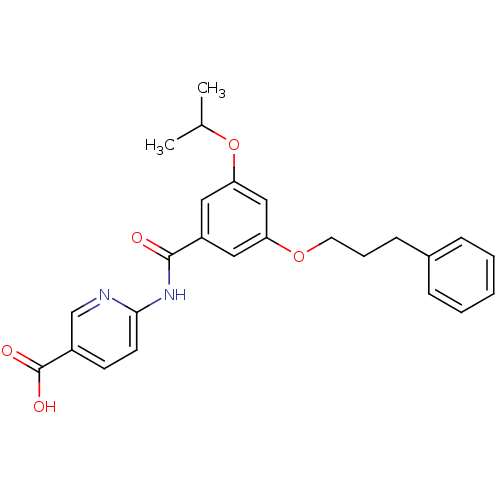
(6-[3-Isopropoxy-5-(3-phenyl-propoxy)-benzoylamino]...)Show SMILES CC(C)Oc1cc(OCCCc2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C25H26N2O5/c1-17(2)32-22-14-20(24(28)27-23-11-10-19(16-26-23)25(29)30)13-21(15-22)31-12-6-9-18-7-4-3-5-8-18/h3-5,7-8,10-11,13-17H,6,9,12H2,1-2H3,(H,29,30)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50165045

(6-{3-Isopropoxy-5-[2-(tetrahydro-pyran-4-yl)-ethox...)Show SMILES CC(C)Oc1cc(OCCC2CCOCC2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C23H28N2O6/c1-15(2)31-20-12-18(22(26)25-21-4-3-17(14-24-21)23(27)28)11-19(13-20)30-10-7-16-5-8-29-9-6-16/h3-4,11-16H,5-10H2,1-2H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a |
AstraZeneca UK
Curated by ChEMBL
| Assay Description
Potency in Glucokinase activation assay |
Bioorg Med Chem Lett 15: 2103-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.087
BindingDB Entry DOI: 10.7270/Q28S4PFN |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344160

((S)-6-(3-(1-methoxypropan-2-yloxy)-5-(4-(methylsul...)Show SMILES COC[C@H](C)Oc1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C24H24N2O8S/c1-15(14-32-2)33-19-10-17(23(27)26-22-9-4-16(13-25-22)24(28)29)11-20(12-19)34-18-5-7-21(8-6-18)35(3,30)31/h4-13,15H,14H2,1-3H3,(H,28,29)(H,25,26,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50183219
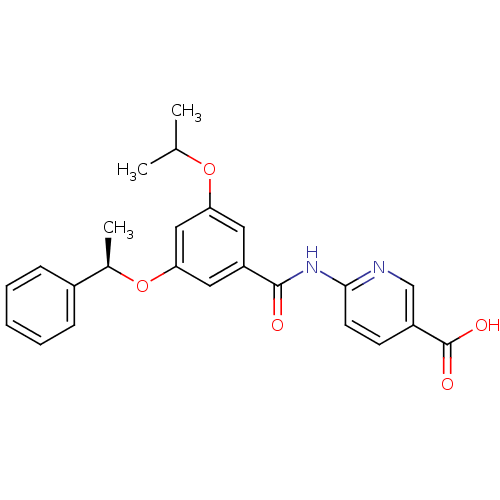
((R)-6-(3-isopropoxy-5-(1-phenylethoxy)benzamido)ni...)Show SMILES CC(C)Oc1cc(O[C@H](C)c2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C24H24N2O5/c1-15(2)30-20-11-19(23(27)26-22-10-9-18(14-25-22)24(28)29)12-21(13-20)31-16(3)17-7-5-4-6-8-17/h4-16H,1-3H3,(H,28,29)(H,25,26,27)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 950 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activitation of glucokinase |
Bioorg Med Chem Lett 16: 2705-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.022
BindingDB Entry DOI: 10.7270/Q28G8K91 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50183220
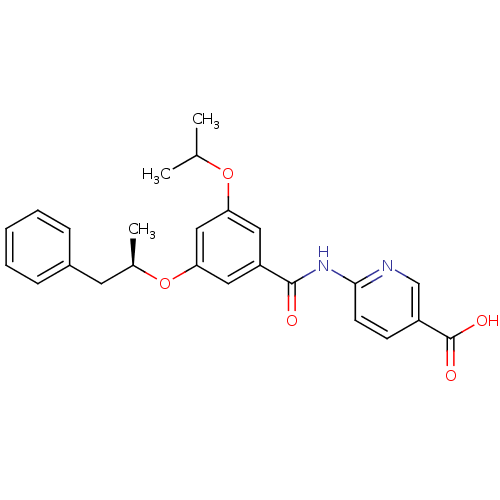
((R)-6-(3-isopropoxy-5-(1-phenylpropan-2-yloxy)benz...)Show SMILES CC(C)Oc1cc(O[C@H](C)Cc2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C25H26N2O5/c1-16(2)31-21-12-20(24(28)27-23-10-9-19(15-26-23)25(29)30)13-22(14-21)32-17(3)11-18-7-5-4-6-8-18/h4-10,12-17H,11H2,1-3H3,(H,29,30)(H,26,27,28)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activitation of glucokinase |
Bioorg Med Chem Lett 16: 2705-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.022
BindingDB Entry DOI: 10.7270/Q28G8K91 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50183222
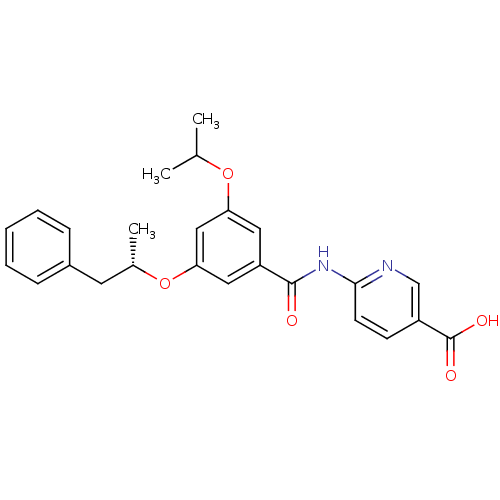
((S)-6-(3-isopropoxy-5-(1-phenylpropan-2-yloxy)benz...)Show SMILES CC(C)Oc1cc(O[C@@H](C)Cc2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C25H26N2O5/c1-16(2)31-21-12-20(24(28)27-23-10-9-19(15-26-23)25(29)30)13-22(14-21)32-17(3)11-18-7-5-4-6-8-18/h4-10,12-17H,11H2,1-3H3,(H,29,30)(H,26,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activitation of glucokinase |
Bioorg Med Chem Lett 16: 2705-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.022
BindingDB Entry DOI: 10.7270/Q28G8K91 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50183221
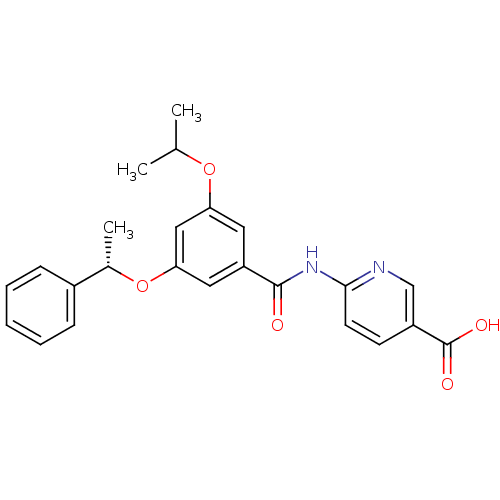
((S)-6-(3-isopropoxy-5-(1-phenylethoxy)benzamido)ni...)Show SMILES CC(C)Oc1cc(O[C@@H](C)c2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C24H24N2O5/c1-15(2)30-20-11-19(23(27)26-22-10-9-18(14-25-22)24(28)29)12-21(13-20)31-16(3)17-7-5-4-6-8-17/h4-16H,1-3H3,(H,28,29)(H,25,26,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activitation of glucokinase |
Bioorg Med Chem Lett 16: 2705-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.022
BindingDB Entry DOI: 10.7270/Q28G8K91 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50183223

((S)-6-(3-isopropoxy-5-(1-methoxypropan-2-yloxy)ben...)Show SMILES COC[C@H](C)Oc1cc(OC(C)C)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C20H24N2O6/c1-12(2)27-16-7-15(8-17(9-16)28-13(3)11-26-4)19(23)22-18-6-5-14(10-21-18)20(24)25/h5-10,12-13H,11H2,1-4H3,(H,24,25)(H,21,22,23)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activitation of glucokinase |
Bioorg Med Chem Lett 16: 2705-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.022
BindingDB Entry DOI: 10.7270/Q28G8K91 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344141
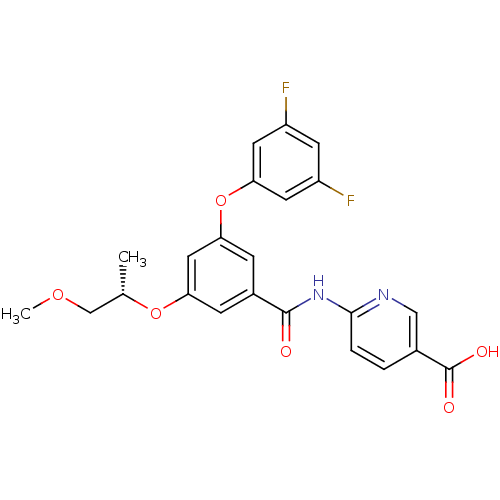
((S)-6-(3-(3,5-difluorophenoxy)-5-(1-methoxypropan-...)Show SMILES COC[C@H](C)Oc1cc(Oc2cc(F)cc(F)c2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C23H20F2N2O6/c1-13(12-31-2)32-18-5-15(22(28)27-21-4-3-14(11-26-21)23(29)30)6-19(10-18)33-20-8-16(24)7-17(25)9-20/h3-11,13H,12H2,1-2H3,(H,29,30)(H,26,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50320996
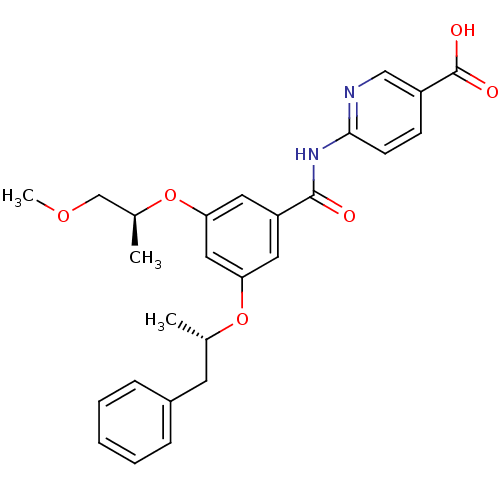
(6-(3-((S)-1-methoxypropan-2-yloxy)-5-((S)-1-phenyl...)Show SMILES COC[C@H](C)Oc1cc(O[C@@H](C)Cc2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C26H28N2O6/c1-17(11-19-7-5-4-6-8-19)33-22-12-21(13-23(14-22)34-18(2)16-32-3)25(29)28-24-10-9-20(15-27-24)26(30)31/h4-10,12-15,17-18H,11,16H2,1-3H3,(H,30,31)(H,27,28,29)/t17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344142

(6-(3-((S)-1-(4-fluorophenyl)propan-2-yloxy)-5-((S)...)Show SMILES COC[C@H](C)Oc1cc(O[C@@H](C)Cc2ccc(F)cc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C26H27FN2O6/c1-16(10-18-4-7-21(27)8-5-18)34-22-11-20(12-23(13-22)35-17(2)15-33-3)25(30)29-24-9-6-19(14-28-24)26(31)32/h4-9,11-14,16-17H,10,15H2,1-3H3,(H,31,32)(H,28,29,30)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344143

(6-(3-((S)-1-(2-methoxyphenyl)propan-2-yloxy)-5-((S...)Show SMILES COC[C@H](C)Oc1cc(O[C@@H](C)Cc2ccccc2OC)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C27H30N2O7/c1-17(11-19-7-5-6-8-24(19)34-4)35-22-12-21(13-23(14-22)36-18(2)16-33-3)26(30)29-25-10-9-20(15-28-25)27(31)32/h5-10,12-15,17-18H,11,16H2,1-4H3,(H,31,32)(H,28,29,30)/t17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344144

(6-(3-((S)-1-(3,5-difluorophenyl)propan-2-yloxy)-5-...)Show SMILES COC[C@H](C)Oc1cc(O[C@@H](C)Cc2cc(F)cc(F)c2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C26H26F2N2O6/c1-15(6-17-7-20(27)11-21(28)8-17)35-22-9-19(10-23(12-22)36-16(2)14-34-3)25(31)30-24-5-4-18(13-29-24)26(32)33/h4-5,7-13,15-16H,6,14H2,1-3H3,(H,32,33)(H,29,30,31)/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344145
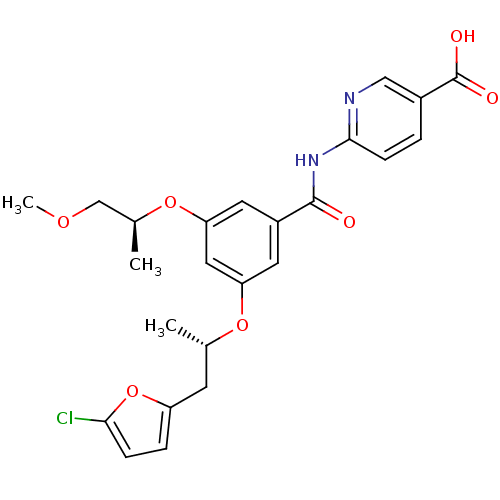
(6-(3-((S)-1-(5-chlorofuran-2-yl)propan-2-yloxy)-5-...)Show SMILES COC[C@H](C)Oc1cc(O[C@@H](C)Cc2ccc(Cl)o2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C24H25ClN2O7/c1-14(8-18-5-6-21(25)34-18)32-19-9-17(10-20(11-19)33-15(2)13-31-3)23(28)27-22-7-4-16(12-26-22)24(29)30/h4-7,9-12,14-15H,8,13H2,1-3H3,(H,29,30)(H,26,27,28)/t14-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344146
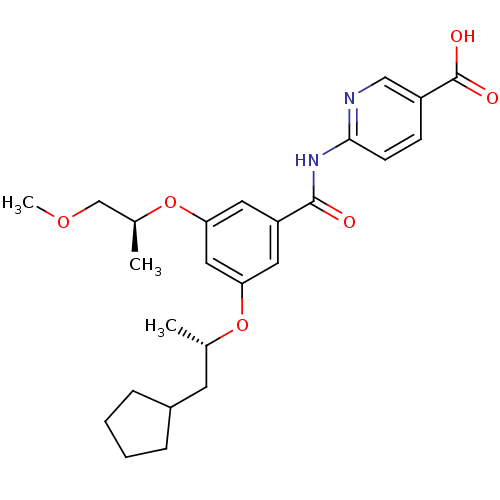
(6-(3-((S)-1-cyclopentylpropan-2-yloxy)-5-((S)-1-me...)Show SMILES COC[C@H](C)Oc1cc(O[C@@H](C)CC2CCCC2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C25H32N2O6/c1-16(10-18-6-4-5-7-18)32-21-11-20(12-22(13-21)33-17(2)15-31-3)24(28)27-23-9-8-19(14-26-23)25(29)30/h8-9,11-14,16-18H,4-7,10,15H2,1-3H3,(H,29,30)(H,26,27,28)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50183222

((S)-6-(3-isopropoxy-5-(1-phenylpropan-2-yloxy)benz...)Show SMILES CC(C)Oc1cc(O[C@@H](C)Cc2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C25H26N2O5/c1-16(2)31-21-12-20(24(28)27-23-10-9-19(15-26-23)25(29)30)13-22(14-21)32-17(3)11-18-7-5-4-6-8-18/h4-10,12-17H,11H2,1-3H3,(H,29,30)(H,26,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344147
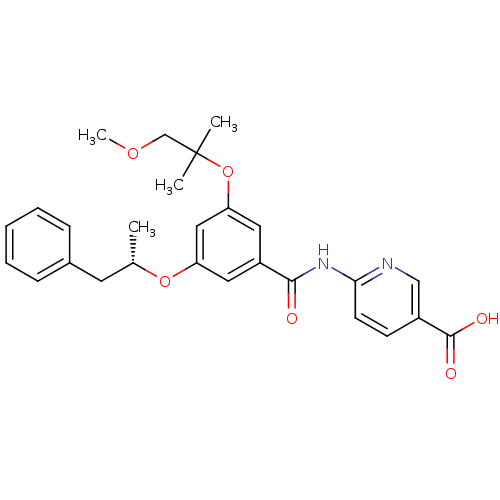
((S)-6-(3-(1-methoxy-2-methylpropan-2-yloxy)-5-(1-p...)Show SMILES COCC(C)(C)Oc1cc(O[C@@H](C)Cc2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C27H30N2O6/c1-18(12-19-8-6-5-7-9-19)34-22-13-21(14-23(15-22)35-27(2,3)17-33-4)25(30)29-24-11-10-20(16-28-24)26(31)32/h5-11,13-16,18H,12,17H2,1-4H3,(H,31,32)(H,28,29,30)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344148

((S)-6-(3-(1-phenylpropan-2-yloxy)-5-(tetrahydro-2H...)Show SMILES C[C@@H](Cc1ccccc1)Oc1cc(OC2CCOCC2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C27H28N2O6/c1-18(13-19-5-3-2-4-6-19)34-23-14-21(15-24(16-23)35-22-9-11-33-12-10-22)26(30)29-25-8-7-20(17-28-25)27(31)32/h2-8,14-18,22H,9-13H2,1H3,(H,31,32)(H,28,29,30)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344149

(6-(3-((S)-1-methoxypropan-2-yloxy)-5-((R)-2-phenyl...)Show SMILES COC[C@H](C)Oc1cc(OC[C@H](C)c2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C26H28N2O6/c1-17(19-7-5-4-6-8-19)15-33-22-11-21(12-23(13-22)34-18(2)16-32-3)25(29)28-24-10-9-20(14-27-24)26(30)31/h4-14,17-18H,15-16H2,1-3H3,(H,30,31)(H,27,28,29)/t17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344150

((S)-6-(3-(2,2-difluoro-2-phenylethoxy)-5-(1-methox...)Show SMILES COC[C@H](C)Oc1cc(OCC(F)(F)c2ccccc2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C25H24F2N2O6/c1-16(14-33-2)35-21-11-18(23(30)29-22-9-8-17(13-28-22)24(31)32)10-20(12-21)34-15-25(26,27)19-6-4-3-5-7-19/h3-13,16H,14-15H2,1-2H3,(H,31,32)(H,28,29,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50344151

(6-(3-(2,3-dihydro-1H-inden-2-yloxy)-5-isopropoxybe...)Show SMILES CC(C)Oc1cc(OC2Cc3ccccc3C2)cc(c1)C(=O)Nc1ccc(cn1)C(O)=O Show InChI InChI=1S/C25H24N2O5/c1-15(2)31-21-11-19(24(28)27-23-8-7-18(14-26-23)25(29)30)12-22(13-21)32-20-9-16-5-3-4-6-17(16)10-20/h3-8,11-15,20H,9-10H2,1-2H3,(H,29,30)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Activation of His-tagged recombinant glucokinase expressed in Escherichia coli using [14C]-glucose substrate by spectrophotometrically |
Bioorg Med Chem Lett 21: 3467-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.093
BindingDB Entry DOI: 10.7270/Q2Z038HG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data