

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50014757 ((4-Chloro-phenyl)-(2-chloro-phenyl)-pyridin-3-yl-m...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485474 (CHEMBL1862838 | DNDI1322027) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485473 (CHEMBL1863499 | DNDI1336037) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485471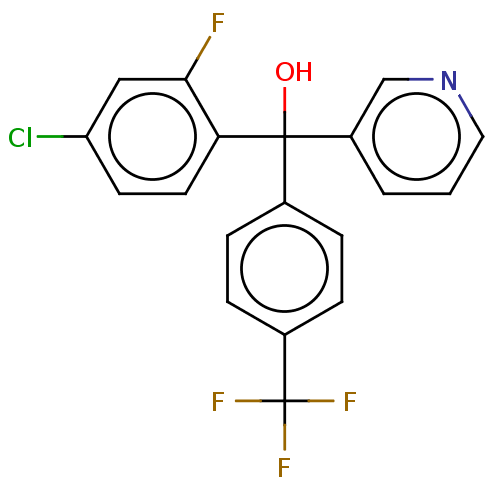 (CHEMBL1863383 | DNDI1335912) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485466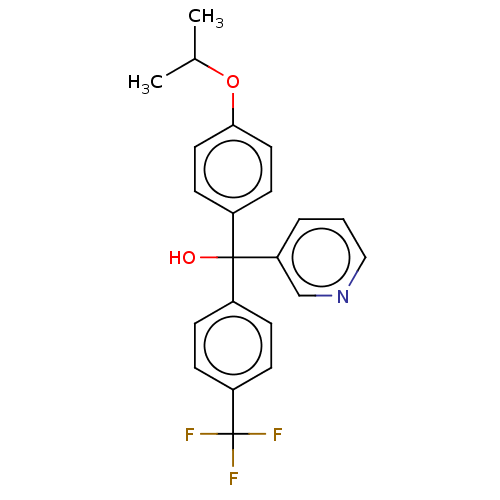 (CHEMBL1863004 | DNDI1335913) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485476 (CHEMBL1863160 | DNDI1294211) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485475 (CHEMBL1863146 | DNDI1336031) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485468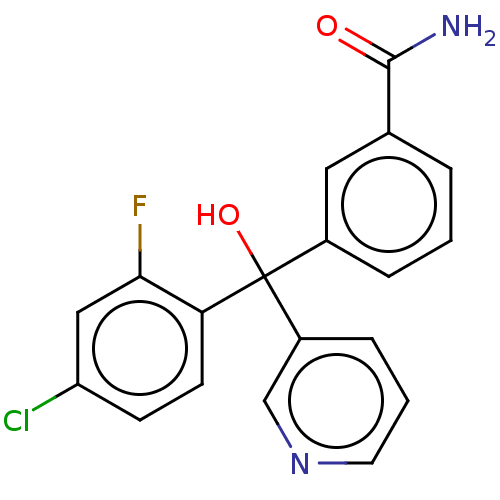 (CHEMBL1863039 | DNDI1467766) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495154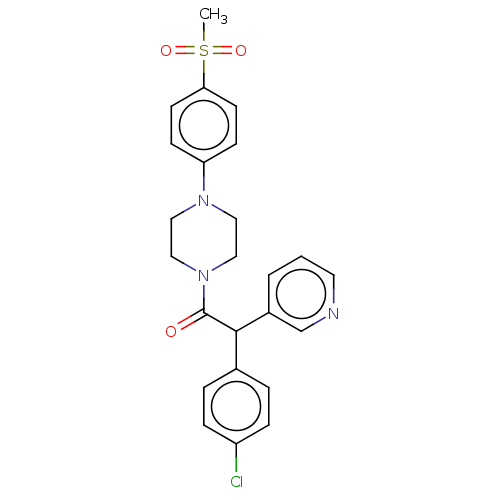 (CHEMBL3104490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485467 (CHEMBL1863325 | DNDI1574842) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485470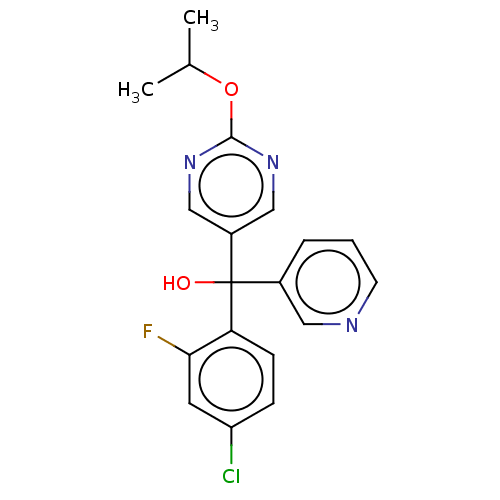 (CHEMBL1863007 | DNDI1342987) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485469 (CHEMBL1863183 | DNDI1268111) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495150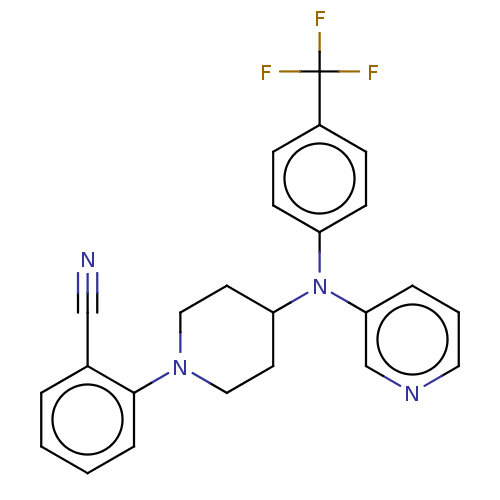 (CHEMBL3104537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495148 (CHEMBL3104479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495146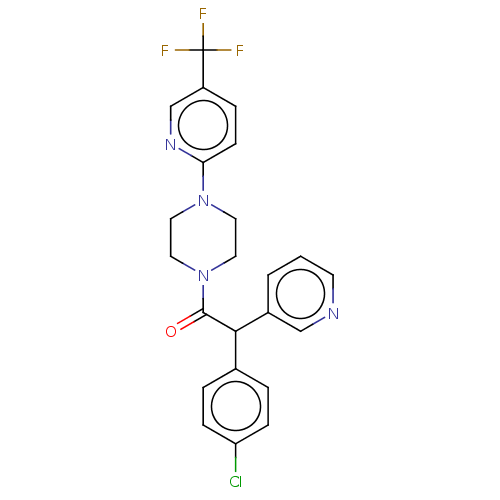 (CHEMBL3104488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50485472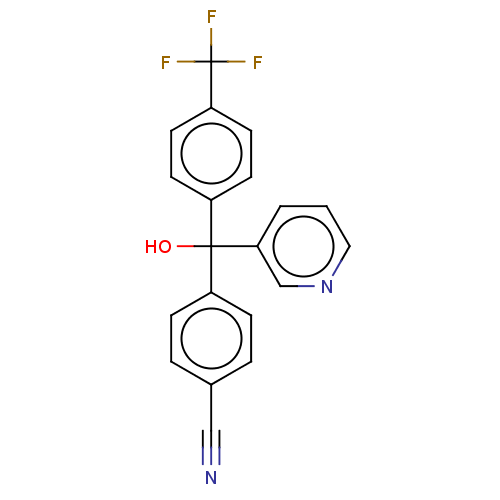 (CHEMBL1863379 | DNDI1330083) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes | J Med Chem 55: 4189-204 (2012) Article DOI: 10.1021/jm2015809 BindingDB Entry DOI: 10.7270/Q2TX3J6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495149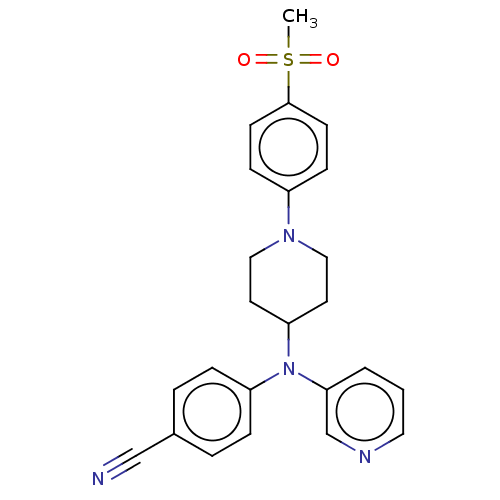 (CHEMBL3104478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495156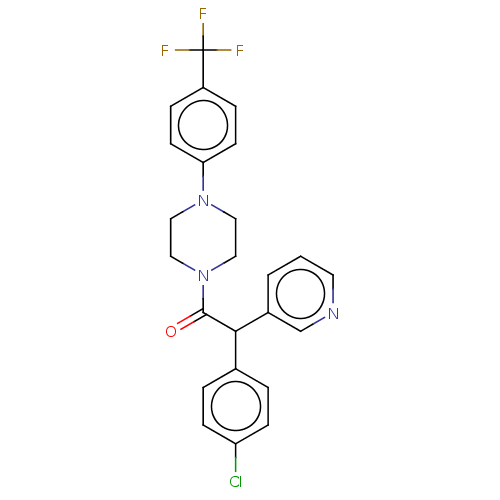 (CHEMBL3104376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495142 (CHEMBL3104473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495152 (CHEMBL3104525) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495151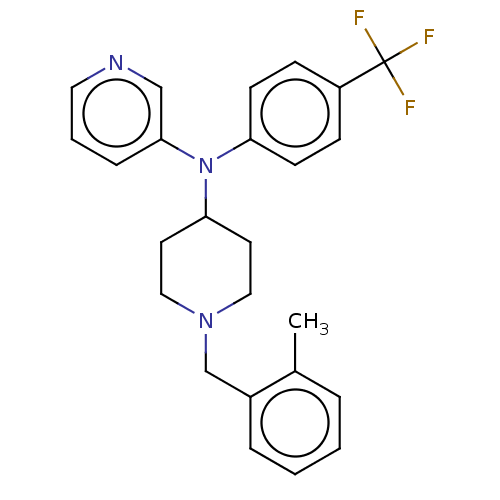 (CHEMBL3104534) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495141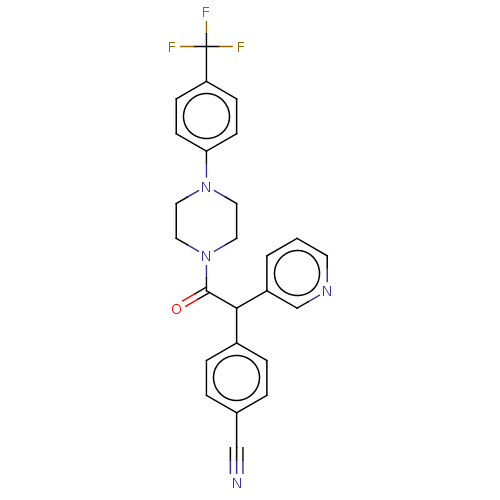 (CHEMBL3104485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495155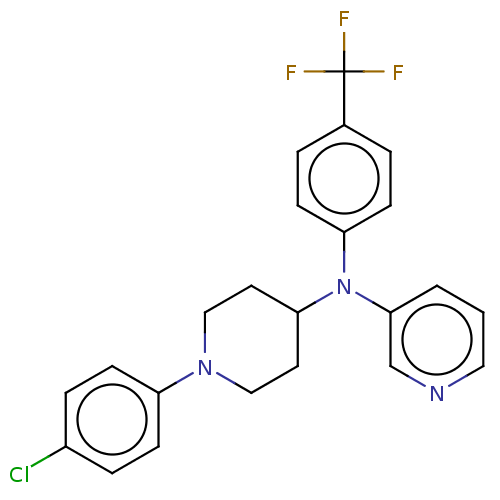 (CHEMBL3104472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495153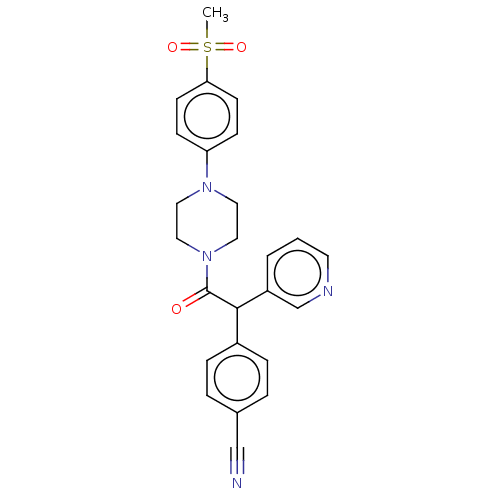 (CHEMBL3104374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495147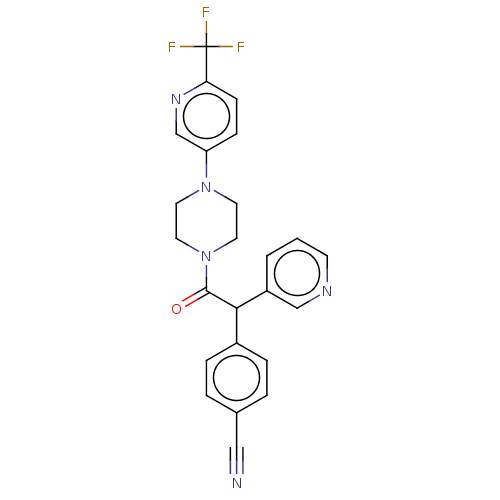 (CHEMBL3104487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495145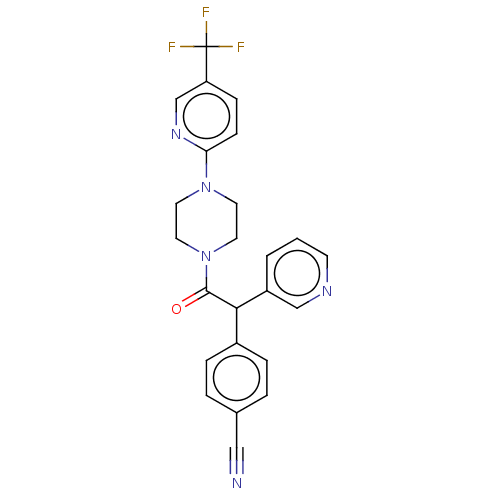 (CHEMBL3104489) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495144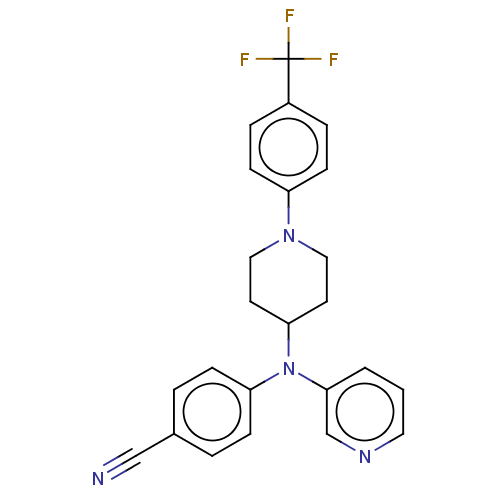 (CHEMBL3104476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495143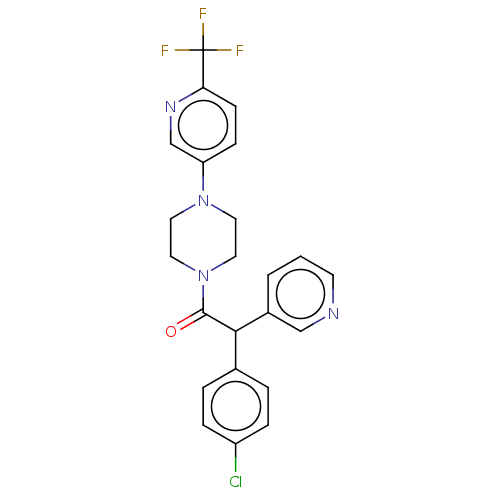 (CHEMBL3104486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative lanosterol 14-alpha-demethylase (Leishmania infantum) | BDBM213823 (EPL-BS1246 (UDO)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 156 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative lanosterol 14-alpha-demethylase (Leishmania infantum) | BDBM213822 (EPL-BS0967 (UDD)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 419 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 19 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Putative lanosterol 14-alpha-demethylase (Leishmania infantum) | BDBM213824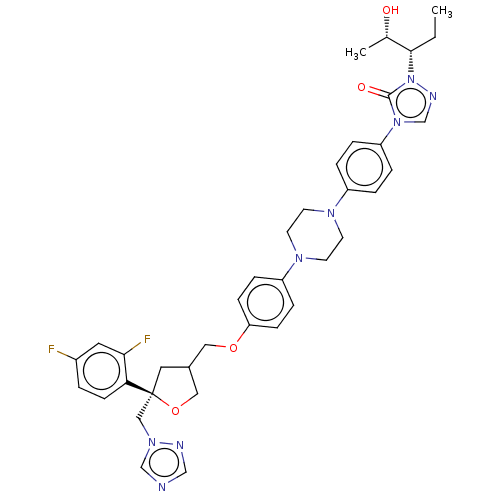 (Posaconazole) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 18 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 26 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 69 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM213824 (Posaconazole) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 18 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM213825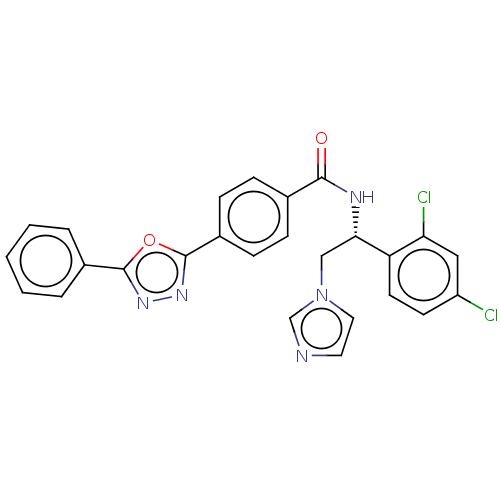 (VNI) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 15 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 32 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||