

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090075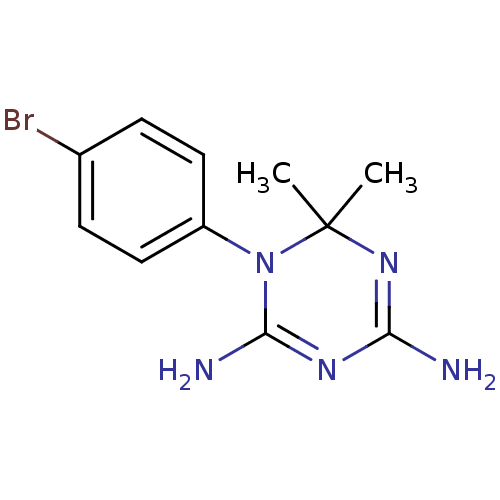 (1-(4-Bromo-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090069 (1-(3,4-Dichloro-phenyl)-6,6-dimethyl-1,6-dihydro-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090062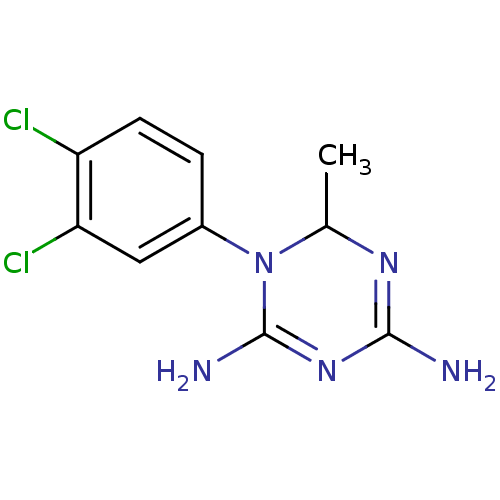 (1-(3,4-Dichloro-phenyl)-6-methyl-1,6-dihydro-[1,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM18792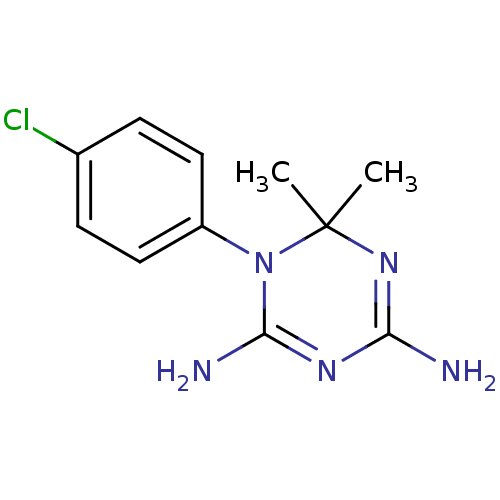 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090056 (1-(3,4-Dichloro-phenyl)-6-phenyl-1,6-dihydro-[1,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090051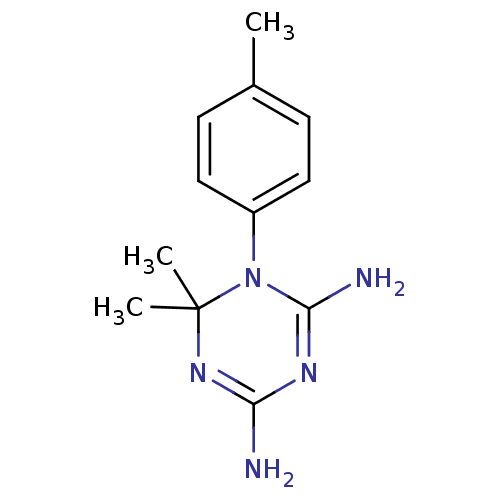 (6,6-Dimethyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090049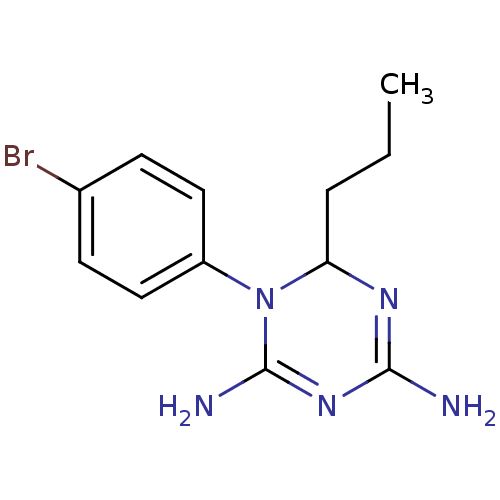 (1-(4-Bromo-phenyl)-6-propyl-1,6-dihydro-[1,3,5]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090060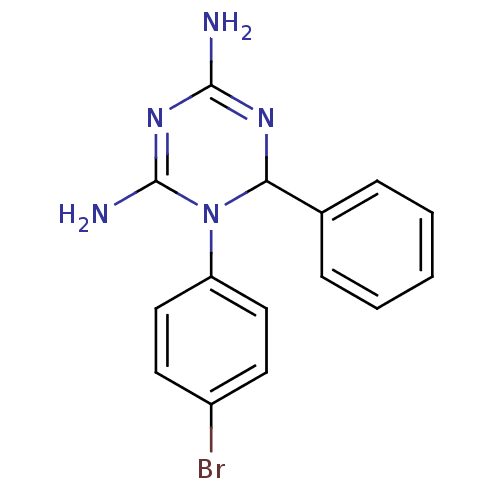 (1-(4-Bromo-phenyl)-6-phenyl-1,6-dihydro-[1,3,5]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090064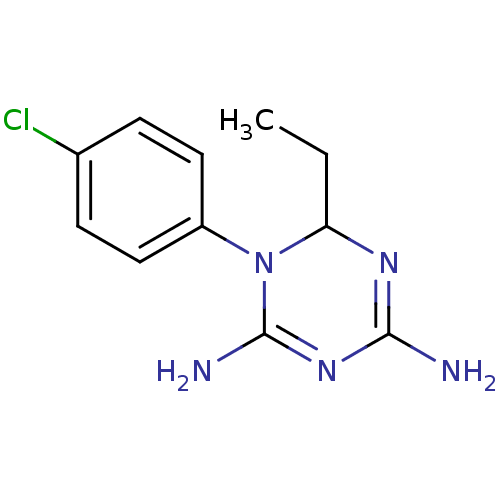 (1-(4-Chloro-phenyl)-6-ethyl-1,6-dihydro-[1,3,5]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090048 (6-Butyl-1-(4-chloro-phenyl)-1,6-dihydro-[1,3,5]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090054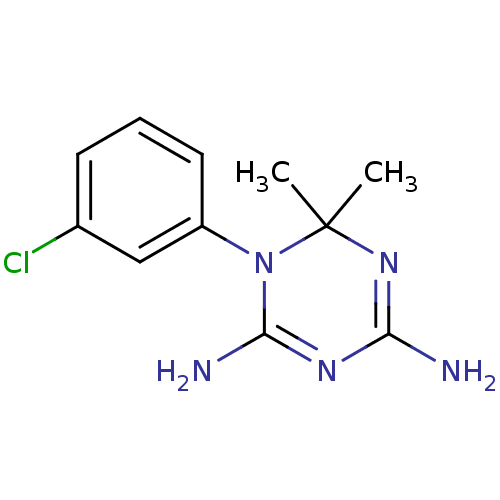 (1-(3-Chloro-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090076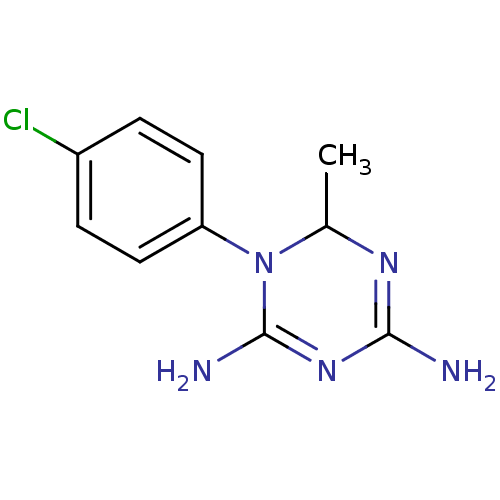 (1-(4-Chloro-phenyl)-6-methyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090070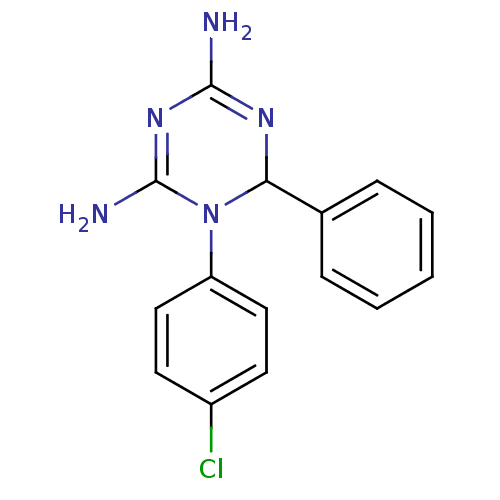 (1-(4-Chloro-phenyl)-6-phenyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090047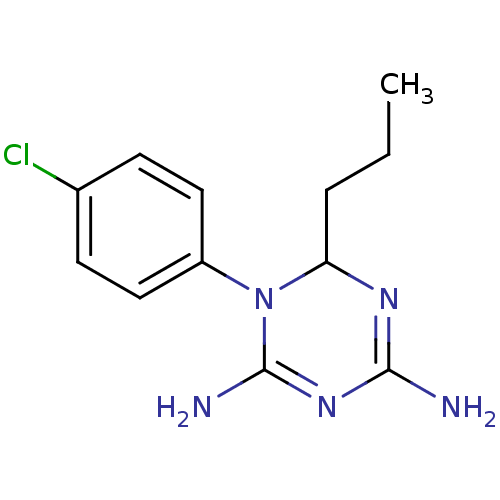 (1-(4-Chloro-phenyl)-6-propyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090050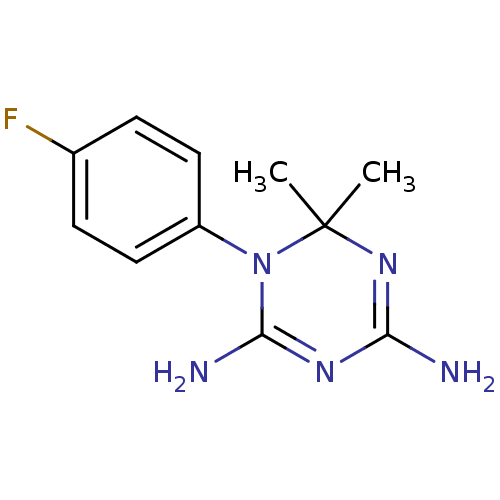 (1-(4-Fluoro-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090066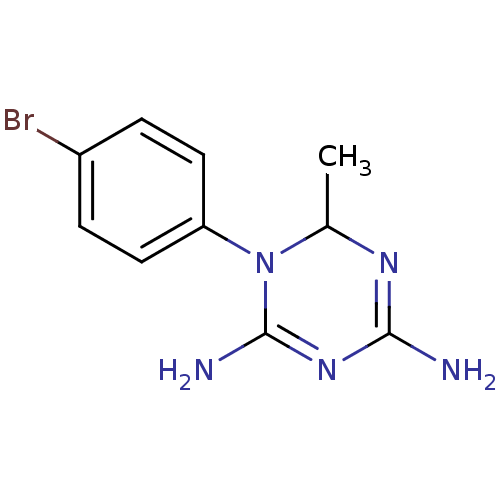 (1-(4-Bromo-phenyl)-6-methyl-1,6-dihydro-[1,3,5]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090061 (1-(4-Bromo-phenyl)-6-ethyl-1,6-dihydro-[1,3,5]tria...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090052 (6-Ethyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine-2,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090068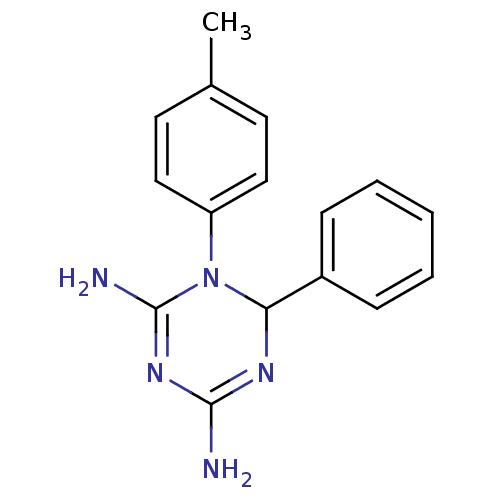 (6-Phenyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine-2,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090063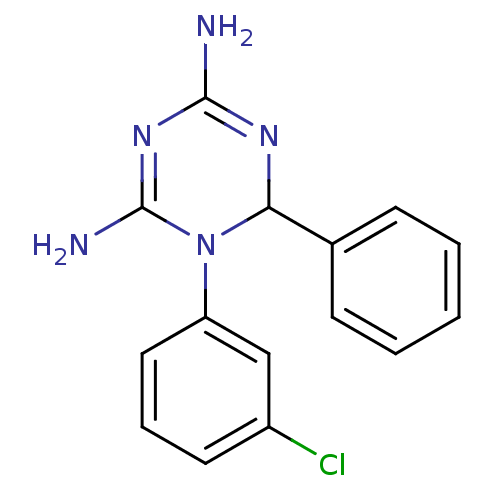 (1-(3-Chloro-phenyl)-6-phenyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki mut) against A16V+S108T Mutant dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090071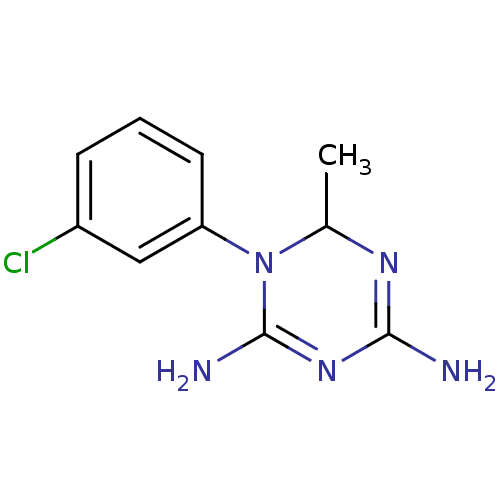 (1-(3-Chloro-phenyl)-6-methyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090056 (1-(3,4-Dichloro-phenyl)-6-phenyl-1,6-dihydro-[1,3,...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki mut) against A16V+S108T Mutant dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090063 (1-(3-Chloro-phenyl)-6-phenyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090058 (6-Propyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine-2,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090062 (1-(3,4-Dichloro-phenyl)-6-methyl-1,6-dihydro-[1,3,...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090067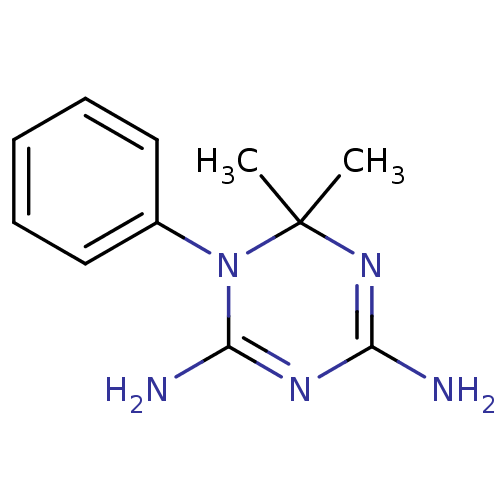 (6,6-Dimethyl-1-phenyl-1,6-dihydro-[1,3,5]triazine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090055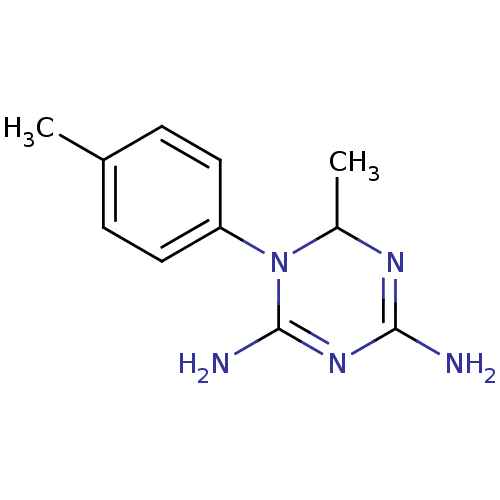 (6-Methyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine-2,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090072 (1-(4-Chloro-phenyl)-1,6-dihydro-[1,3,5]triazine-2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090053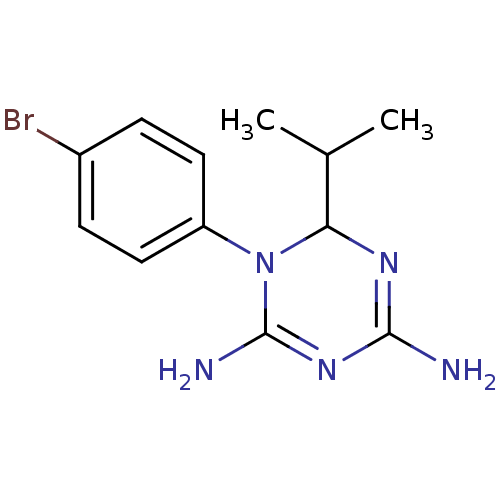 (1-(4-Bromo-phenyl)-6-isopropyl-1,6-dihydro-[1,3,5]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090071 (1-(3-Chloro-phenyl)-6-methyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090070 (1-(4-Chloro-phenyl)-6-phenyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki mut) against A16V+S108T Mutant dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090065 (1-(4-Chloro-phenyl)-6-isopropyl-1,6-dihydro-[1,3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090060 (1-(4-Bromo-phenyl)-6-phenyl-1,6-dihydro-[1,3,5]tri...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki mut) against A16V+S108T Mutant dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090047 (1-(4-Chloro-phenyl)-6-propyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki mut) against A16V+S108T Mutant dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090049 (1-(4-Bromo-phenyl)-6-propyl-1,6-dihydro-[1,3,5]tri...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki mut) against A16V+S108T Mutant dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090076 (1-(4-Chloro-phenyl)-6-methyl-1,6-dihydro-[1,3,5]tr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090052 (6-Ethyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine-2,4-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090069 (1-(3,4-Dichloro-phenyl)-6,6-dimethyl-1,6-dihydro-[...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090059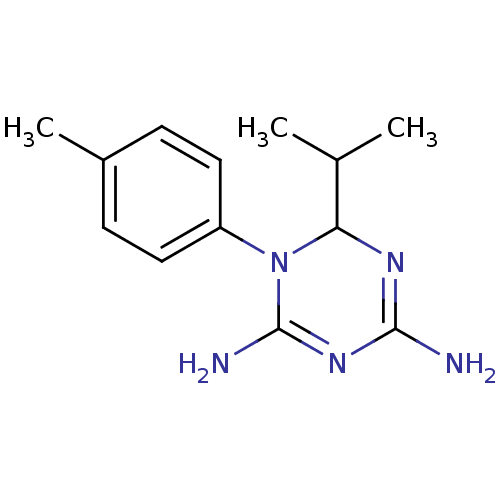 (6-Isopropyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090048 (6-Butyl-1-(4-chloro-phenyl)-1,6-dihydro-[1,3,5]tri...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki mut) against A16V+S108T Mutant dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090068 (6-Phenyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine-2,4...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki mut) against A16V+S108T Mutant dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090055 (6-Methyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine-2,4...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090058 (6-Propyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine-2,4...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090064 (1-(4-Chloro-phenyl)-6-ethyl-1,6-dihydro-[1,3,5]tri...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090061 (1-(4-Bromo-phenyl)-6-ethyl-1,6-dihydro-[1,3,5]tria...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090066 (1-(4-Bromo-phenyl)-6-methyl-1,6-dihydro-[1,3,5]tri...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090073 (1-(4-Fluoro-phenyl)-1,6-dihydro-[1,3,5]triazine-2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum) | BDBM50090057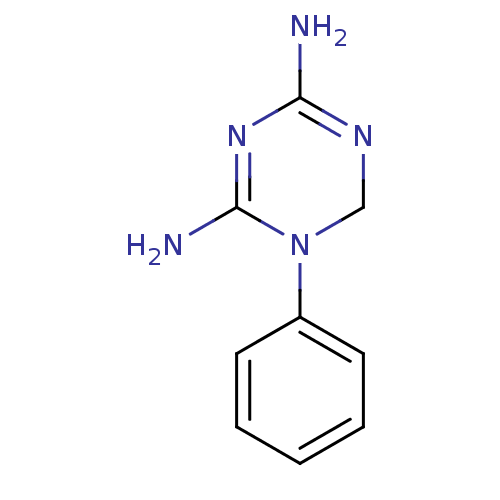 (1-Phenyl-1,6-dihydro-[1,3,5]triazine-2,4-diamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Evaluated for inhibition constant (Ki wt) against Wild-type dihydrofolate reductase of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090054 (1-(3-Chloro-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [A16V,S108T] (Plasmodium falciparum) | BDBM50090073 (1-(4-Fluoro-phenyl)-1,6-dihydro-[1,3,5]triazine-2,...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 469 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition constant (Ki mut) against A16V+S108T Mutant DHFRs of Plasmodium falciparum | J Med Chem 43: 2738-44 (2000) BindingDB Entry DOI: 10.7270/Q2P55MRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |