

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331048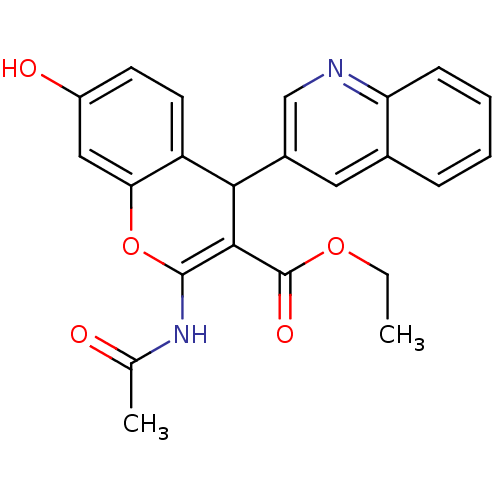 (CHEMBL1277437 | ethyl 2-acetamido-7-hydroxy-4-(qui...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449010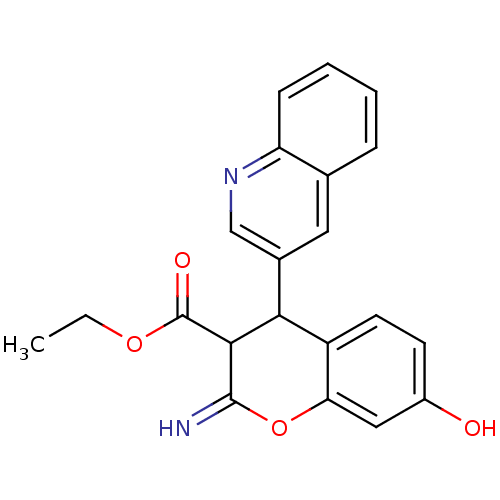 (CHEMBL3125960) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449026 (CHEMBL3125936) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449000 (CHEMBL3125942) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449033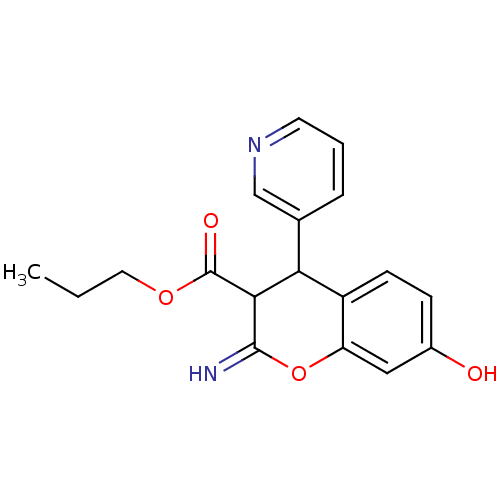 (CHEMBL3125949) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449029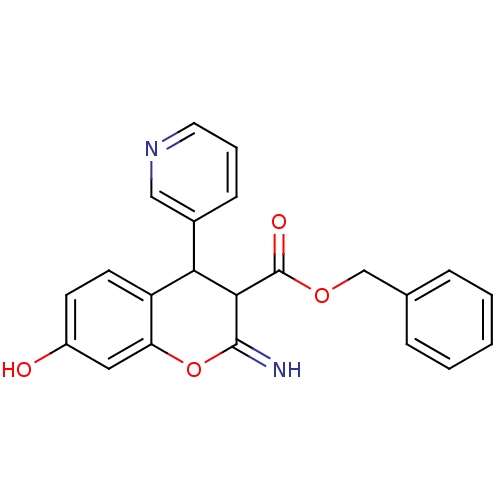 (CHEMBL3125954) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449011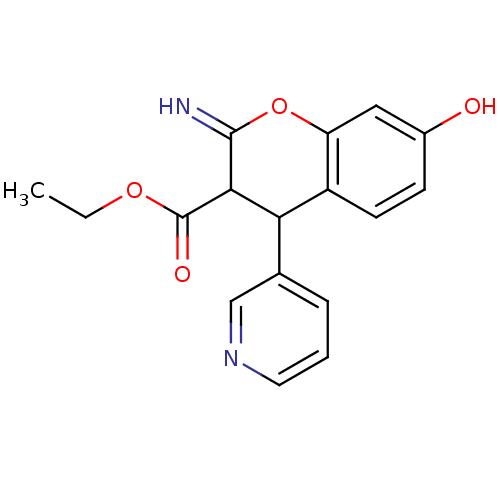 (CHEMBL1604159) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449032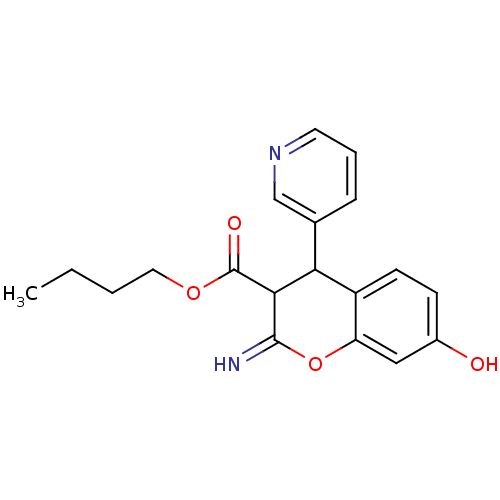 (CHEMBL3125950) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449008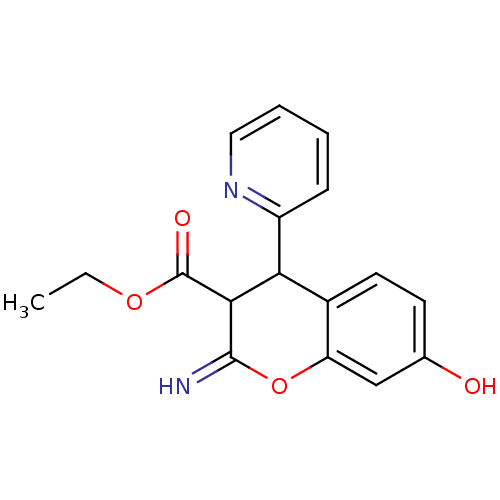 (CHEMBL3125965) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449007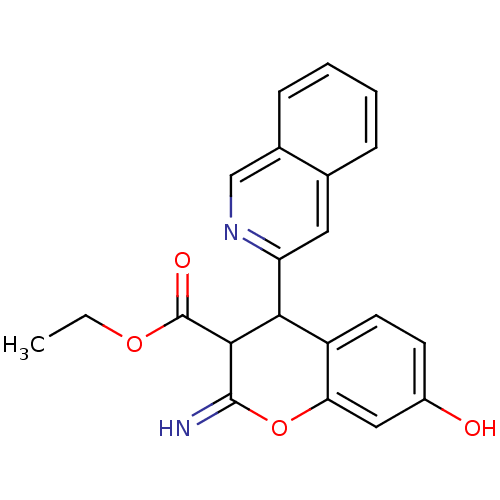 (CHEMBL3125966) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449004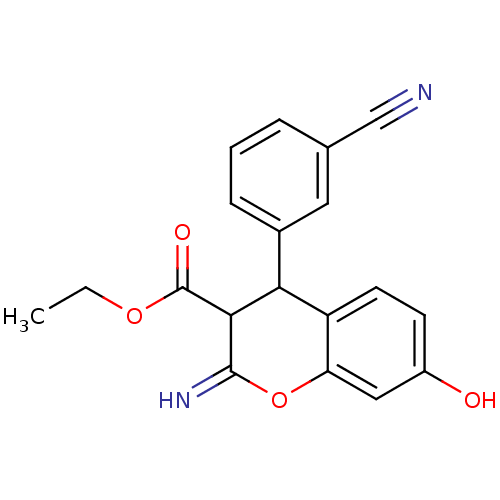 (CHEMBL3125937) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449001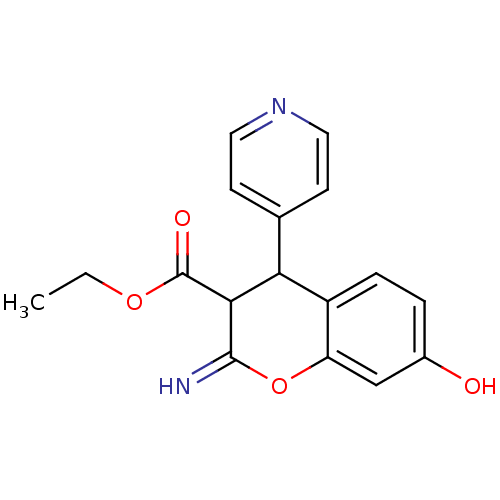 (CHEMBL3125941) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449030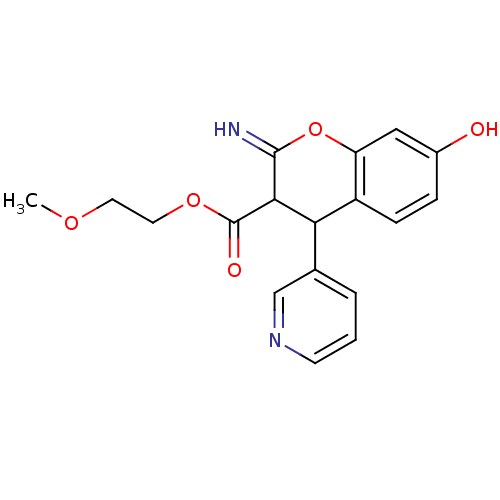 (CHEMBL3125952) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449034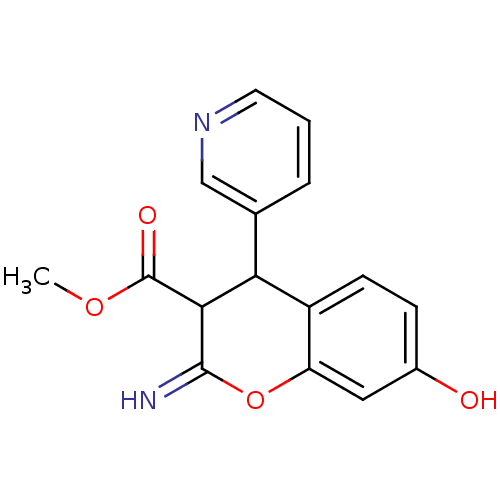 (CHEMBL3125948) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50448996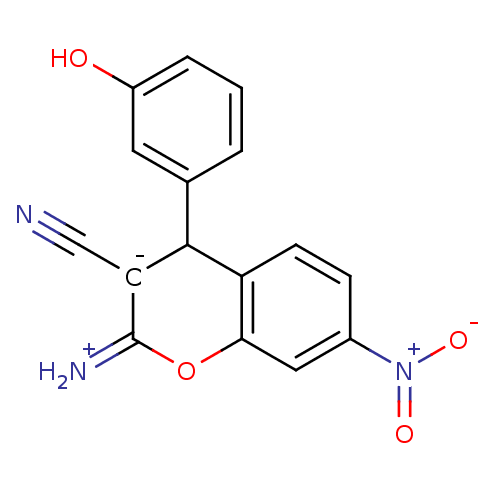 (CHEMBL3125931) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50448998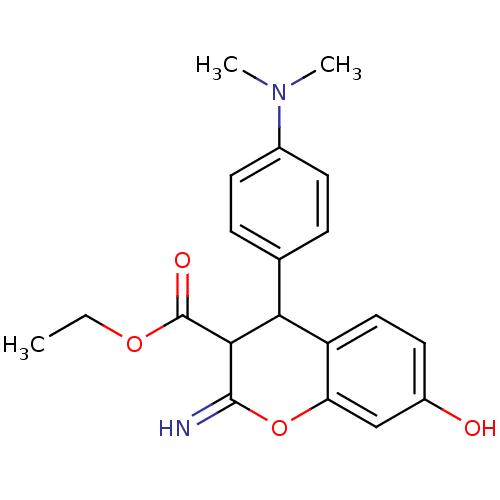 (CHEMBL3125944) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449028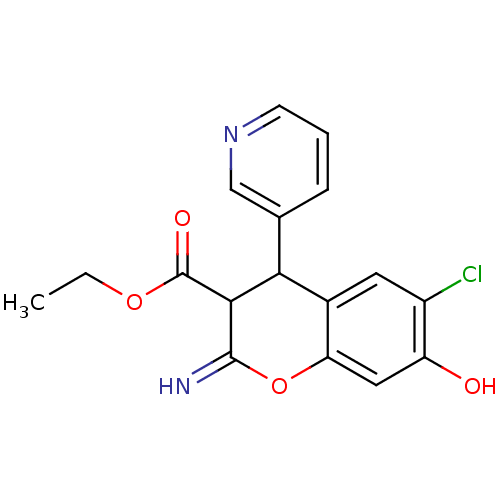 (CHEMBL3125932) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50448997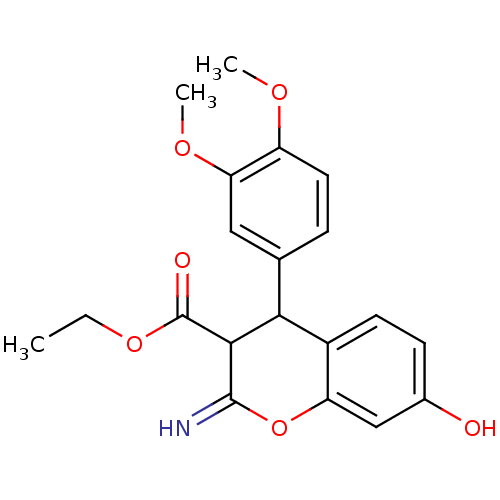 (CHEMBL3125945) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50448999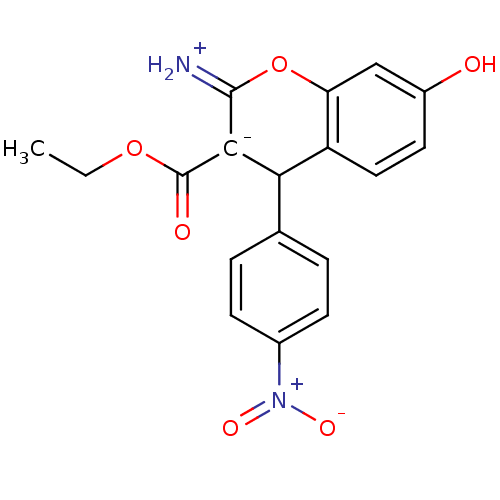 (CHEMBL1338272) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449027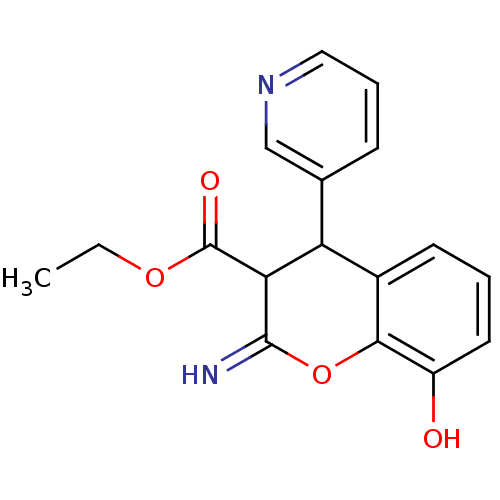 (CHEMBL3125934) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449002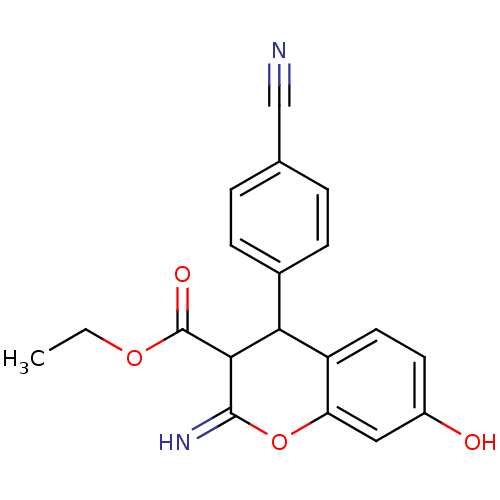 (CHEMBL3125940) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449031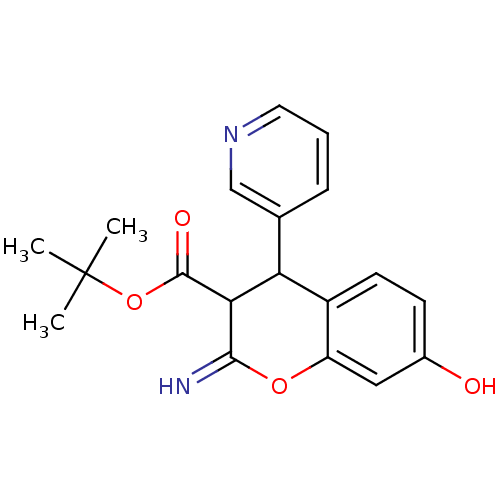 (CHEMBL3125951) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449003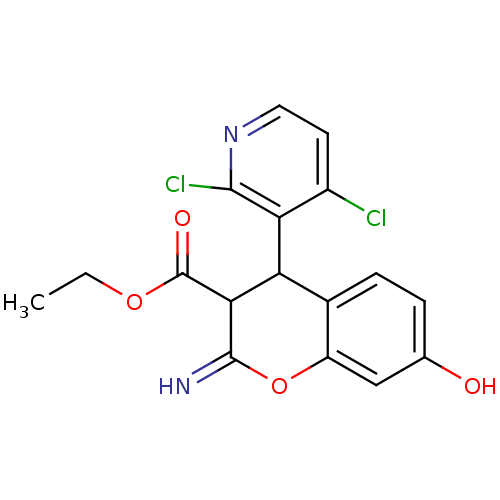 (CHEMBL3125938) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449005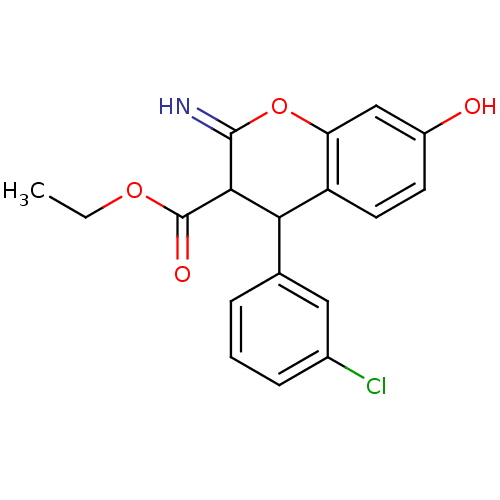 (CHEMBL1511289) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449006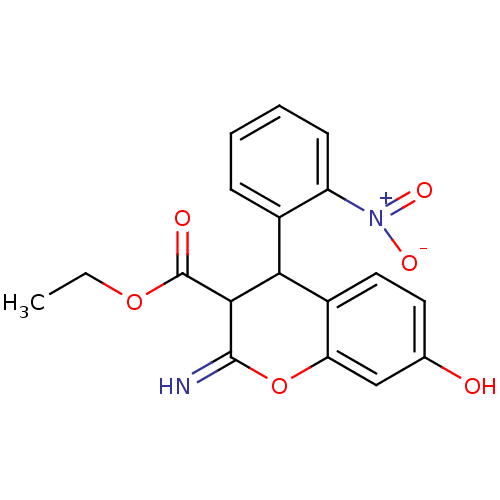 (CHEMBL3125967) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449009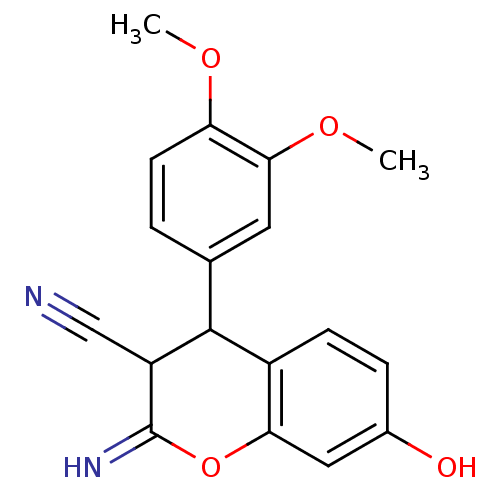 (CHEMBL1303063) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449024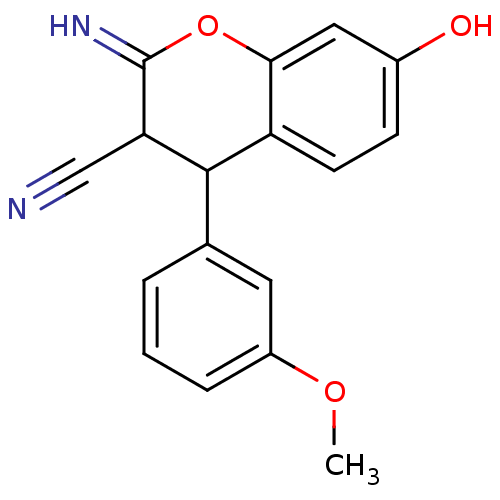 (CHEMBL3125961) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449025 (CHEMBL1276538) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449014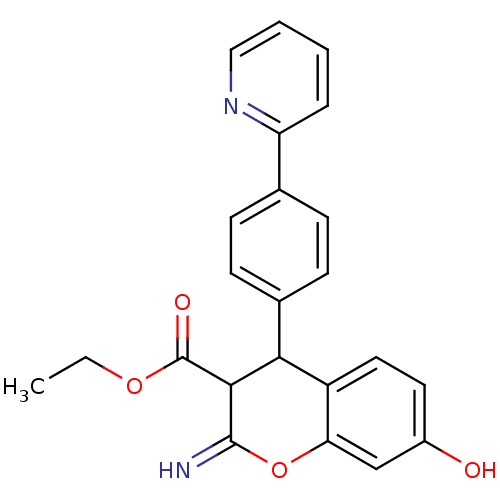 (CHEMBL3125943) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449013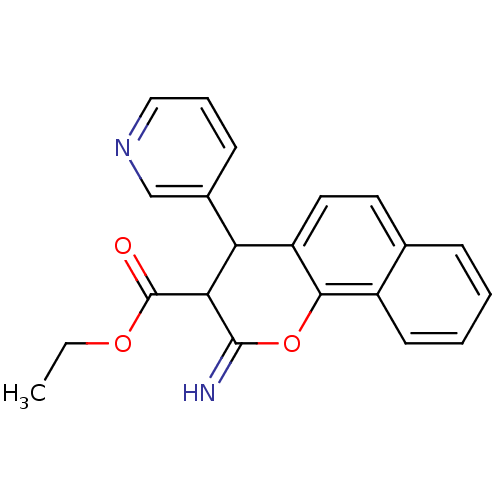 (CHEMBL3125935) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449012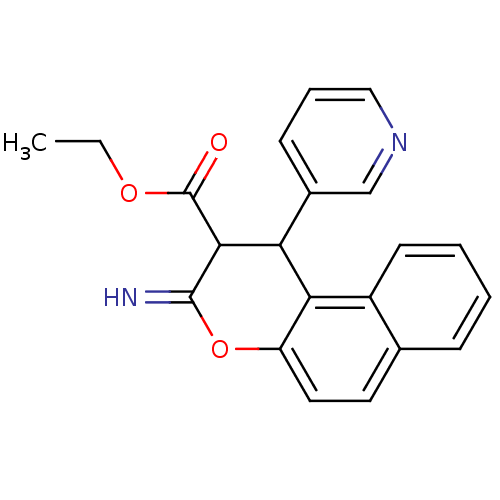 (CHEMBL1406709) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449023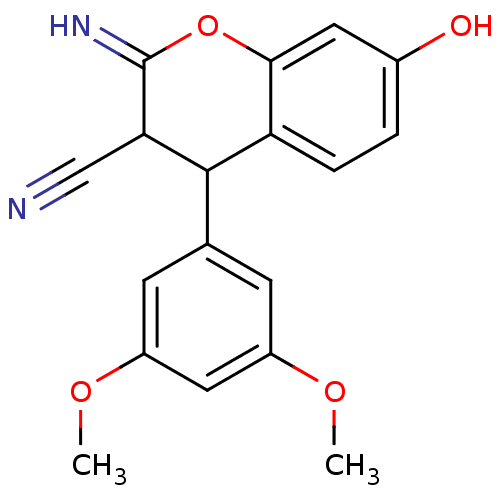 (CHEMBL3125962) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449022 (CHEMBL1403937) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449021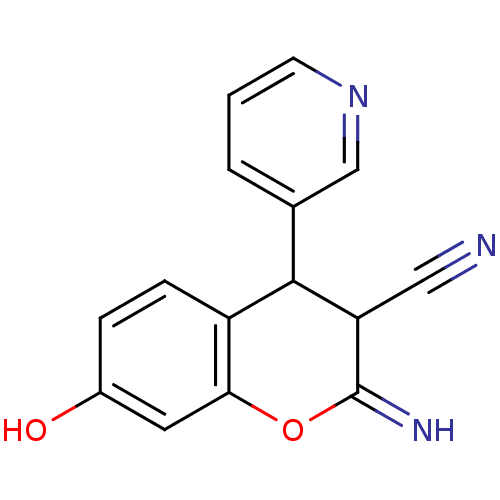 (CHEMBL1556216) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449020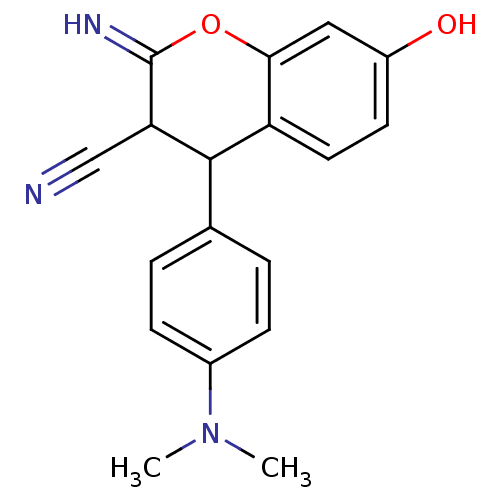 (CHEMBL3125963) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449019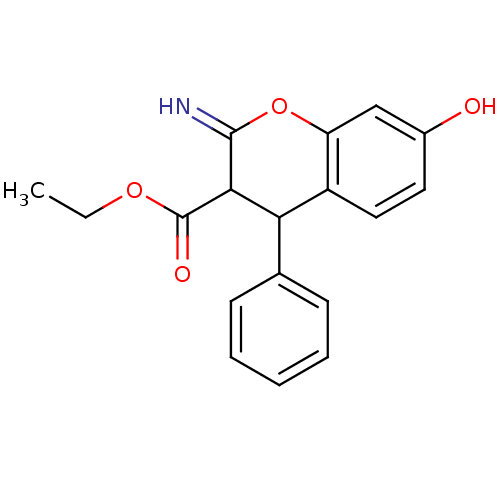 (CHEMBL1603587) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449018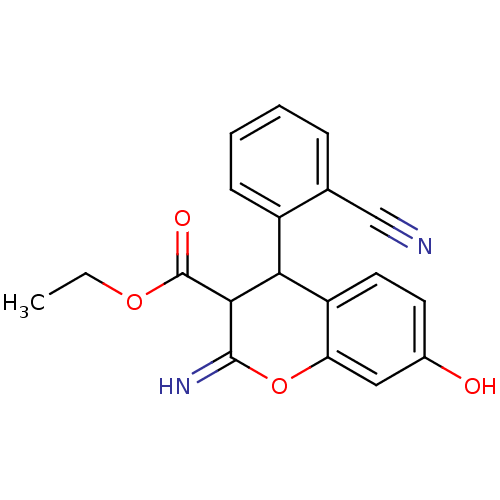 (CHEMBL3125964) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449015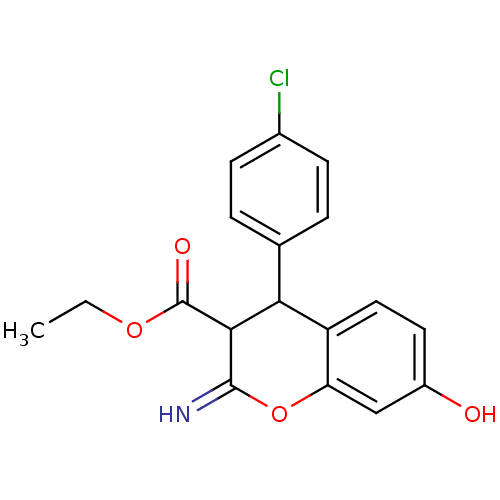 (CHEMBL3125939) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449016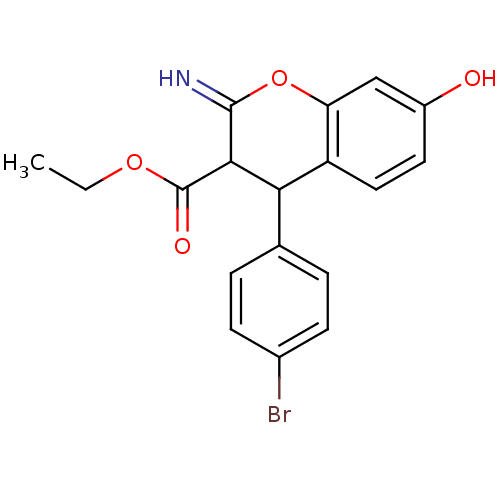 (CHEMBL1435420) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449017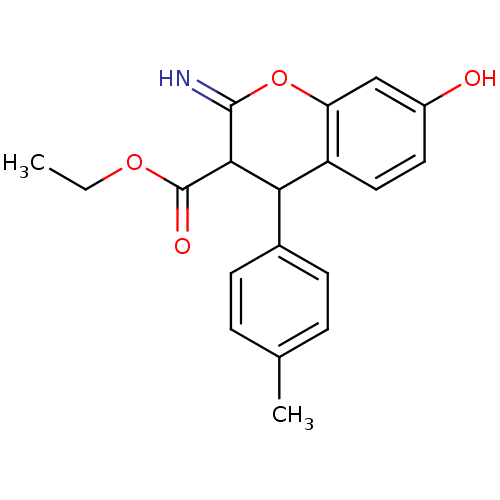 (CHEMBL1604644) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50035218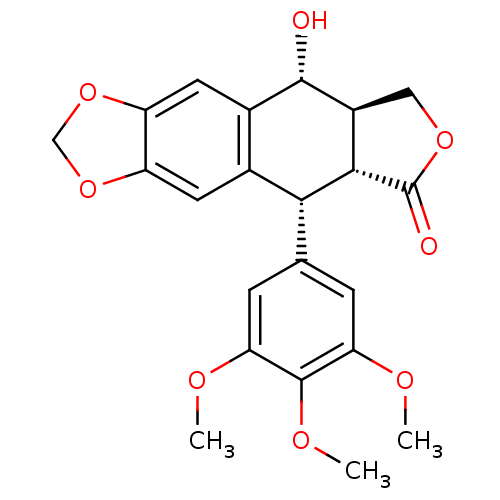 (CHEMBL61 | PODOFILOX | Podophyllinic acid lactone ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville campus) Curated by ChEMBL | Assay Description Inhibition of human glucocorticoid receptor | Antimicrob Agents Chemother 53: 2824-33 (2009) Article DOI: 10.1128/AAC.01568-08 BindingDB Entry DOI: 10.7270/Q2QV3MSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM50345406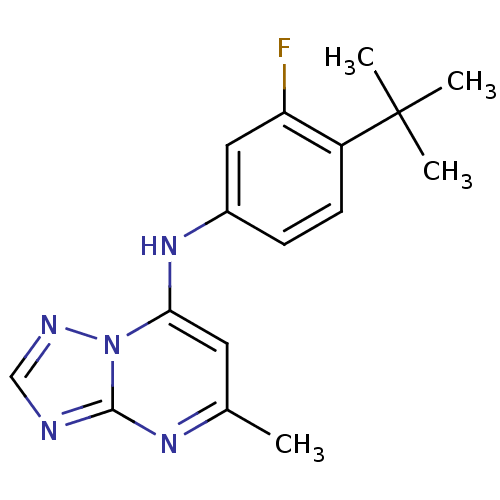 (CHEMBL1784574 | N-(4-tert-Butyl-3-fluorophenyl)-5-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant Plasmodium falciparum dihydroorotate dehydrogenase expressed in Escherichia coli using L-dihydroorotate as subs... | J Med Chem 54: 3935-49 (2011) Article DOI: 10.1021/jm200265b BindingDB Entry DOI: 10.7270/Q2FN16HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM24400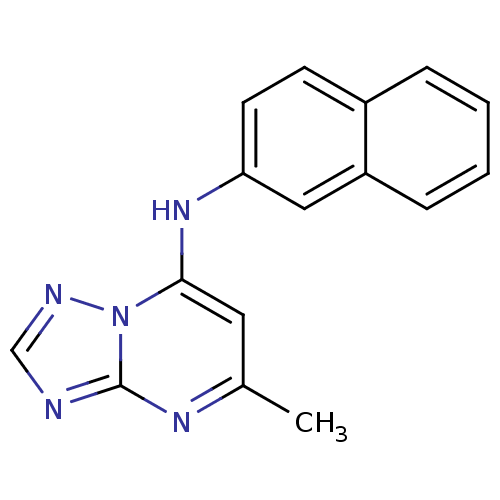 (5-methyl-N-(naphthalen-2-yl)-[1,2,4]triazolo[1,5-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 8.0 | 20 |
University of Washington at Seattle | Assay Description The assays were carried out by using a colorimetric DCIP method, which uses the colorimetric reagent 2, 6-dichlorophenolindophenol as the final elect... | J Med Chem 52: 1864-72 (2009) Article DOI: 10.1021/jm801343r BindingDB Entry DOI: 10.7270/Q2QN653C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM24413 (JMC521864 Compund DSM2 | N-(anthracen-2-yl)-5-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | 8.0 | 20 |
University of Washington at Seattle | Assay Description The assays were carried out by using a colorimetric DCIP method, which uses the colorimetric reagent 2, 6-dichlorophenolindophenol as the final elect... | J Med Chem 52: 1864-72 (2009) Article DOI: 10.1021/jm801343r BindingDB Entry DOI: 10.7270/Q2QN653C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM50345441 (5-Methyl-N-(5,6,7,8-tetrahydronaphthalen-2yl)-[1,2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant Plasmodium falciparum dihydroorotate dehydrogenase expressed in Escherichia coli using L-dihydroorotate as subs... | J Med Chem 54: 3935-49 (2011) Article DOI: 10.1021/jm200265b BindingDB Entry DOI: 10.7270/Q2FN16HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM28843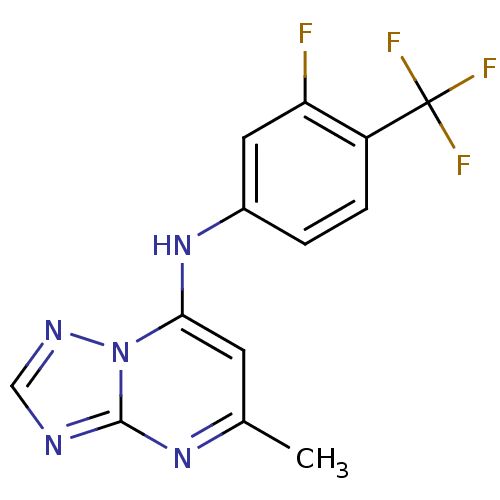 (N-[3-fluoro-4-(trifluoromethyl)phenyl]-5-methyl-[1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description The assays were carried out by using a colorimetric DCIP method, which uses the colorimetric reagent 2, 6-dichlorophenolindophenol as the final elect... | J Med Chem 52: 1864-72 (2009) Article DOI: 10.1021/jm801343r BindingDB Entry DOI: 10.7270/Q2QN653C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM50345420 (CHEMBL1784561 | N-(4-tert-Butylphenyl)-5-methyl-[1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant Plasmodium falciparum dihydroorotate dehydrogenase expressed in Escherichia coli using L-dihydroorotate as subs... | J Med Chem 54: 3935-49 (2011) Article DOI: 10.1021/jm200265b BindingDB Entry DOI: 10.7270/Q2FN16HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM50345385 (CHEMBL1784580 | N-(Benzo[b]thiophen-5-yl)-5-methyl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant Plasmodium falciparum dihydroorotate dehydrogenase expressed in Escherichia coli using L-dihydroorotate as subs... | J Med Chem 54: 3935-49 (2011) Article DOI: 10.1021/jm200265b BindingDB Entry DOI: 10.7270/Q2FN16HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50305505 (CHEMBL227855 | OZ-209 | dispiro[adamantane-2,2'-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | Bioorg Med Chem Lett 20: 563-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.088 BindingDB Entry DOI: 10.7270/Q2RJ4JKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM50345414 (CHEMBL1784567 | N-(4-Pentafluorosulfenylphenyl)-5-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant Plasmodium falciparum dihydroorotate dehydrogenase expressed in Escherichia coli using L-dihydroorotate as subs... | J Med Chem 54: 3935-49 (2011) Article DOI: 10.1021/jm200265b BindingDB Entry DOI: 10.7270/Q2FN16HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 347 total ) | Next | Last >> |