

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175251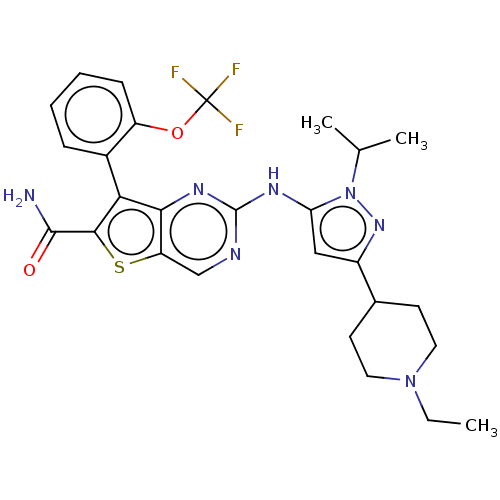 (US9115140, I-123) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175246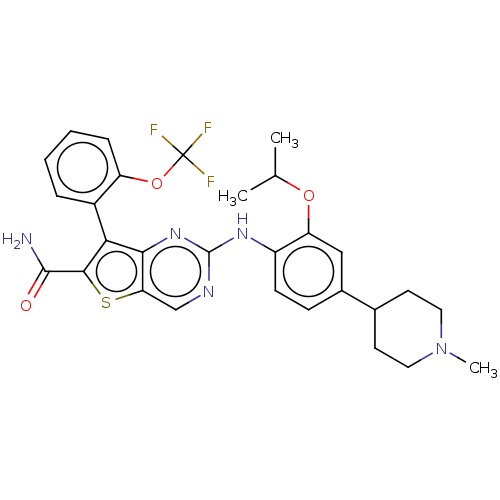 (US9115140, I-26) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175253 (US9115140, I-131) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175250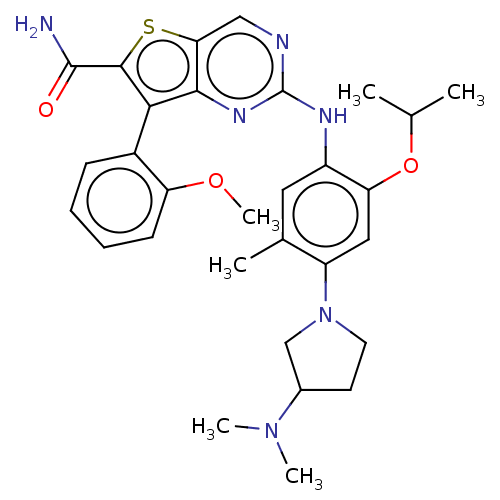 (US9115140, I-118) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175250 (US9115140, I-118) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389047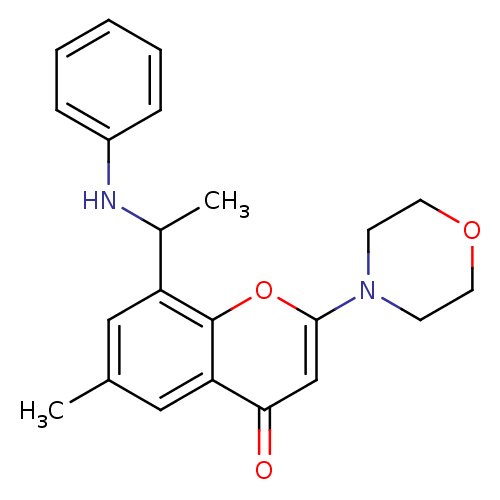 (CHEMBL2064328) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175248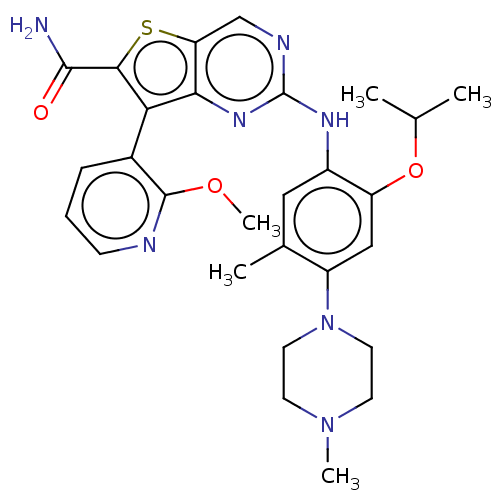 (US9115140, I-100) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175248 (US9115140, I-100) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175253 (US9115140, I-131) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389047 (CHEMBL2064328) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at T308 residue expressed in PTEN-deficient human PC3 cells after 2 hrs by Western blot ana... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50365890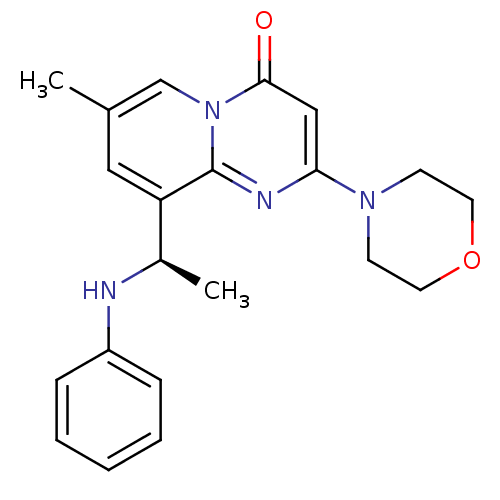 (CHEMBL1957875) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at T308 residue expressed in PTEN-deficient human PC3 cells after 2 hrs by Western blot ana... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175255 (US9115140, I-159) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175251 (US9115140, I-123) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389048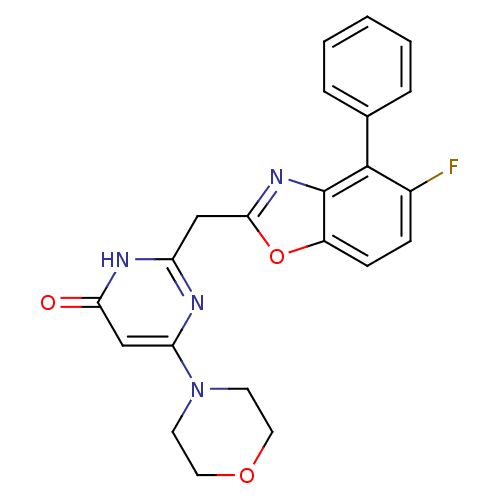 (CHEMBL2064417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175245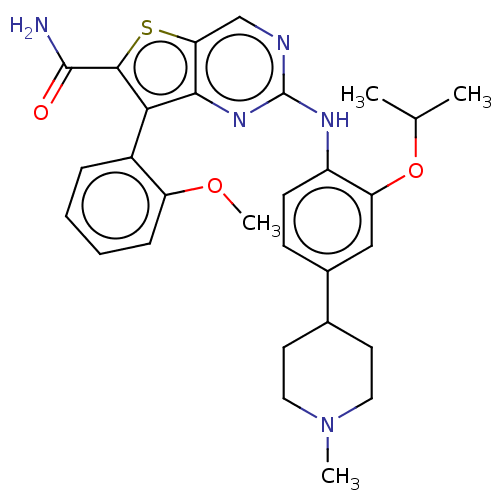 (US9115140, I-16) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175247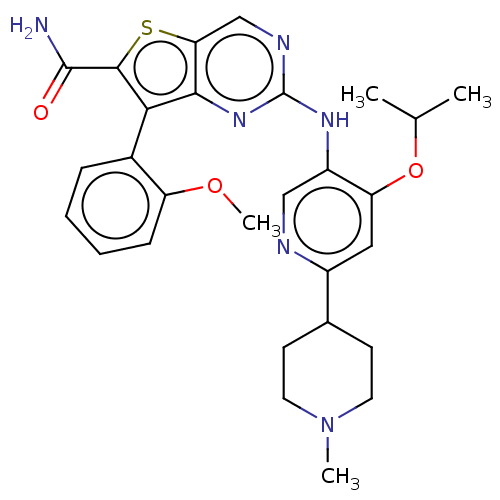 (US9115140, I-38) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175257 (US9115140, I-172) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389069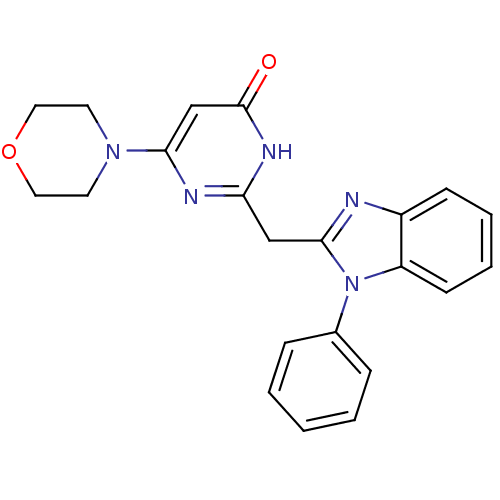 (CHEMBL2064344) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389048 (CHEMBL2064417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of N-terminus poly-His tagged human PI3Kbeta expressed in baculovirus infected insect S21 cells coexpressing p85alpha using PI(4,5)P2 as s... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389072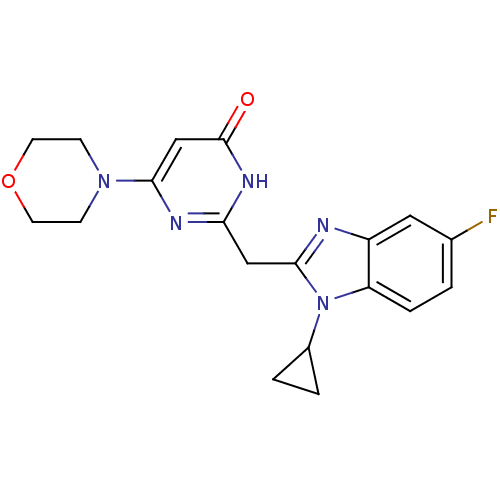 (CHEMBL2064341) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175255 (US9115140, I-159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389067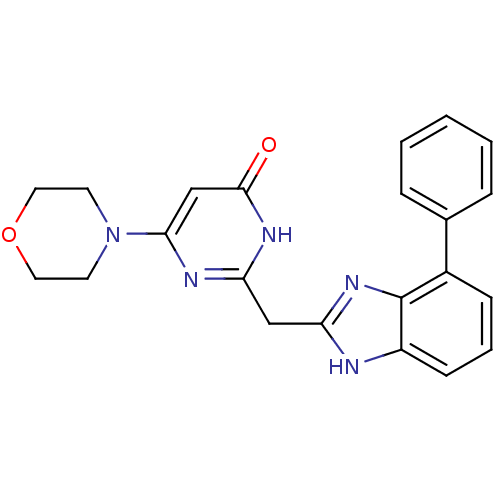 (CHEMBL2064346) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of N-terminus poly-His tagged human PI3Kbeta expressed in baculovirus infected insect S21 cells coexpressing p85alpha using PI(4,5)P2 as s... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50365890 (CHEMBL1957875) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50365890 (CHEMBL1957875) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in mouse MEF-3T3 cells after 0.5 to 2 hrs by Western blot analy... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175249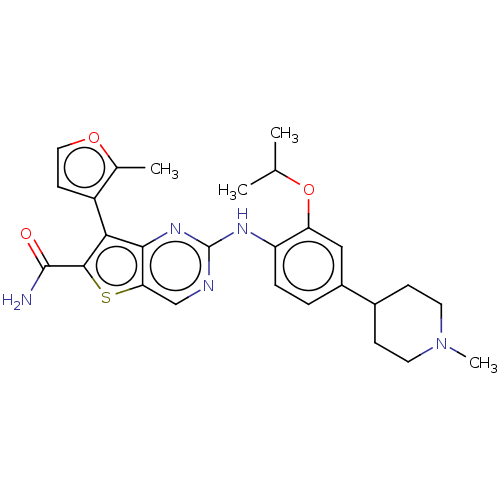 (US9115140, I-115) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175257 (US9115140, I-172) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389054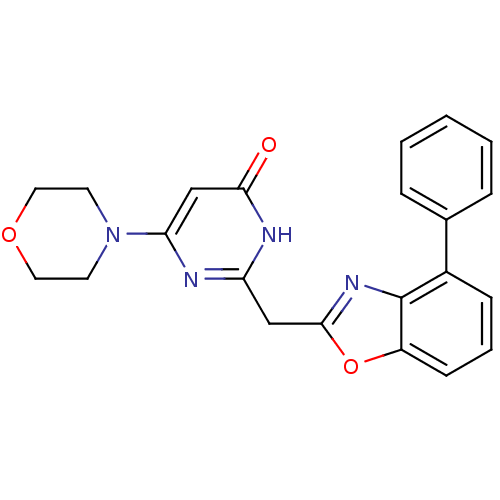 (CHEMBL2064411) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM60576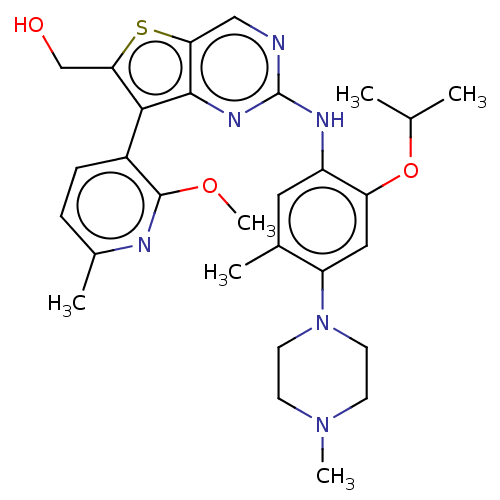 (US9115140, I-191) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175244 (US9115140, I-13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389041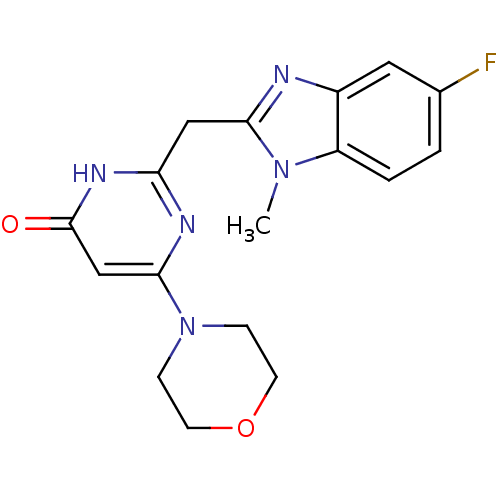 (CHEMBL2064333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175249 (US9115140, I-115) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175254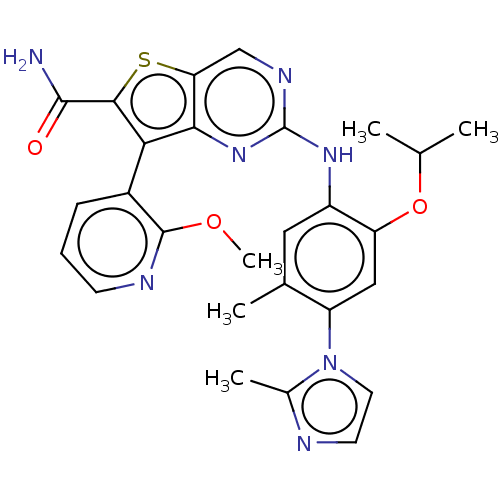 (US9115140, I-132) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389065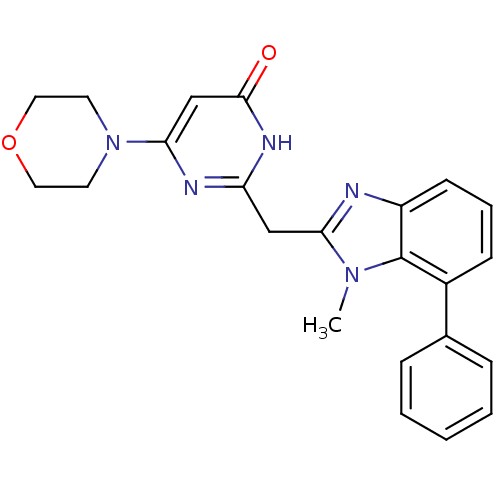 (CHEMBL2064348) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389054 (CHEMBL2064411) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of N-terminus poly-His tagged human PI3Kbeta expressed in baculovirus infected insect S21 cells coexpressing p85alpha using PI(4,5)P2 as s... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50389070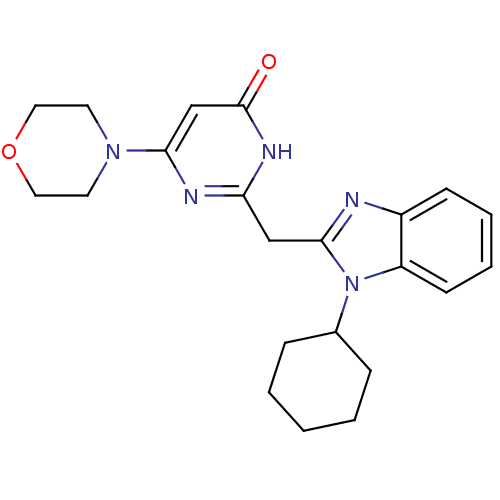 (CHEMBL2064343) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of poly-His tagged human PI3Kdelta expressed in baculovirus infected insect sf9 cells coexpressing p85alpha using PI(4,5)P2 as substrate p... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389052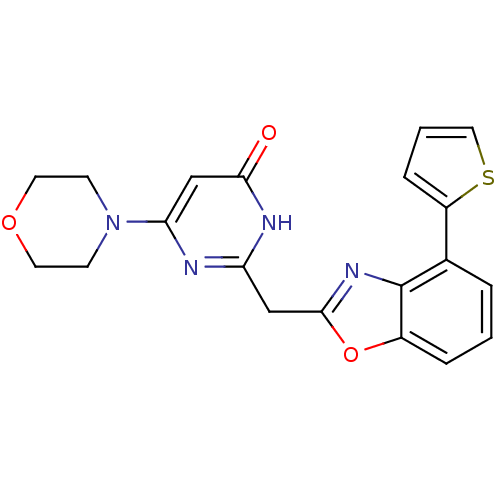 (CHEMBL2064413) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175258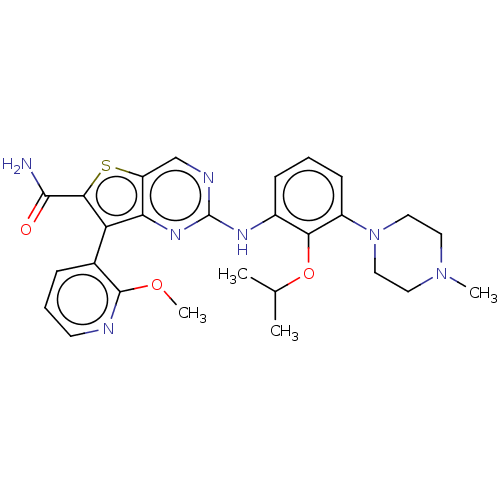 (US9115140, I-176) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389062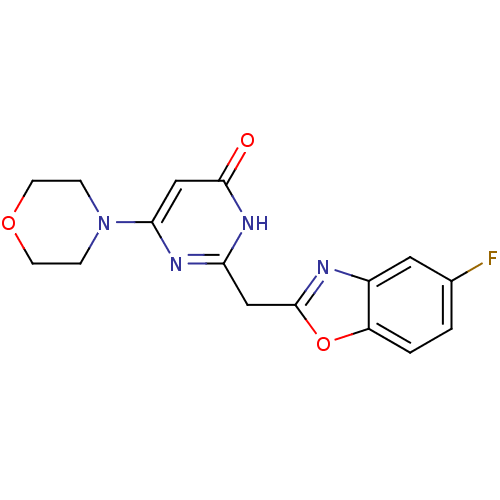 (CHEMBL2064352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389073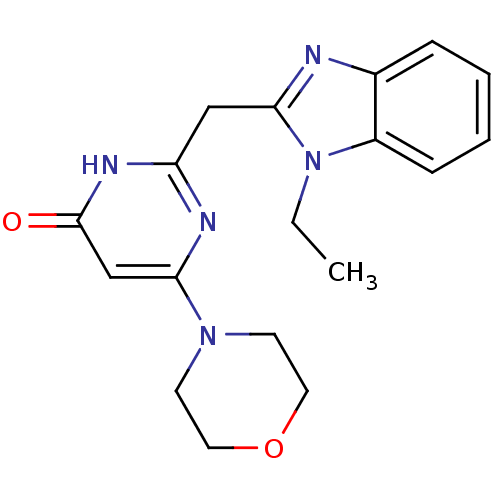 (CHEMBL2064340) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389066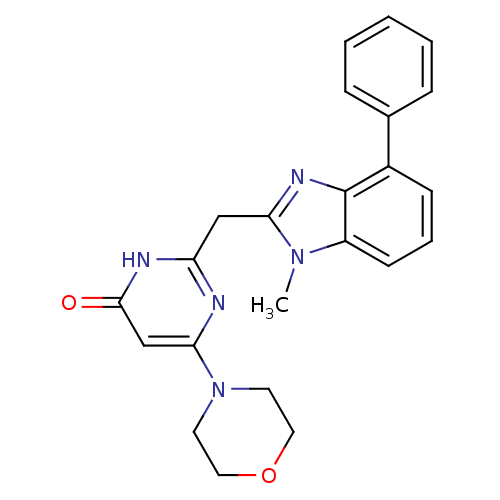 (CHEMBL2064347) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of human PI3Kbeta-mediated Akt phosphorylation at Ser473 residue expressed in PTEN-deficient human PC3 cells by chemiluminescence assay | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175260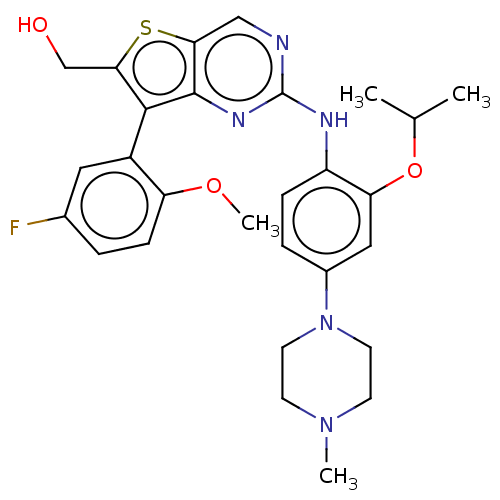 (US9115140, I-202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175243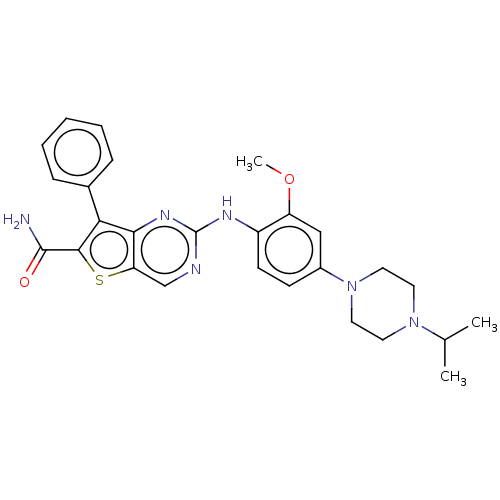 (US9115140, I-7) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389047 (CHEMBL2064328) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of N-terminus poly-His tagged human PI3Kbeta expressed in baculovirus infected insect S21 cells coexpressing p85alpha using PI(4,5)P2 as s... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175260 (US9115140, I-202) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389072 (CHEMBL2064341) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of N-terminus poly-His tagged human PI3Kbeta expressed in baculovirus infected insect S21 cells coexpressing p85alpha using PI(4,5)P2 as s... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389042 (CHEMBL2064331) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of N-terminus poly-His tagged human PI3Kbeta expressed in baculovirus infected insect S21 cells coexpressing p85alpha using PI(4,5)P2 as s... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50365890 (CHEMBL1957875) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of N-terminus poly-His tagged human PI3Kbeta expressed in baculovirus infected insect S21 cells coexpressing p85alpha using PI(4,5)P2 as s... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50389046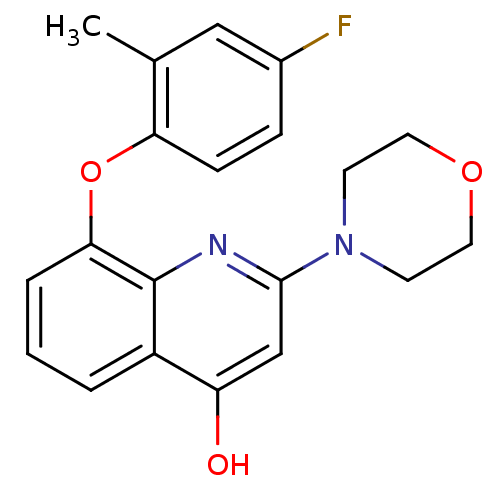 (CHEMBL2064327) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of N-terminus poly-His tagged human PI3Kbeta expressed in baculovirus infected insect S21 cells coexpressing p85alpha using PI(4,5)P2 as s... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175256 (US9115140, I-164) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50389069 (CHEMBL2064344) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research& Development Curated by ChEMBL | Assay Description Inhibition of poly-His tagged human PI3Kdelta expressed in baculovirus infected insect sf9 cells coexpressing p85alpha using PI(4,5)P2 as substrate p... | J Med Chem 55: 4788-805 (2012) Article DOI: 10.1021/jm300241b BindingDB Entry DOI: 10.7270/Q2R78G88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 325 total ) | Next | Last >> |