

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50579647 (CHEMBL5090920) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Jack bean alpha-mannosidase by Lineweaver-Burk plot | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00036 BindingDB Entry DOI: 10.7270/Q28G8QK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50579646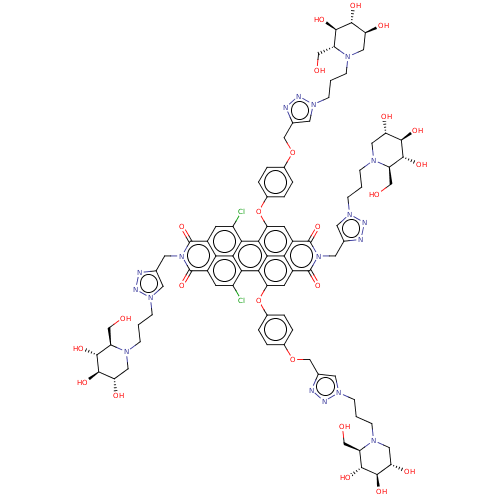 (CHEMBL5078012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Jack bean alpha-mannosidase by Lineweaver-Burk plot | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00036 BindingDB Entry DOI: 10.7270/Q28G8QK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169013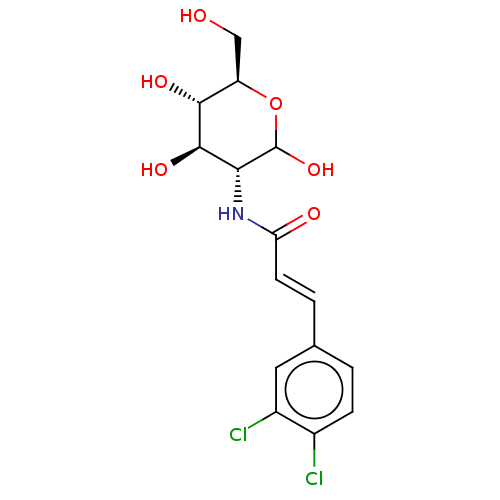 (CHEMBL3805703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) assessed as formation of G6P by continuou... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433027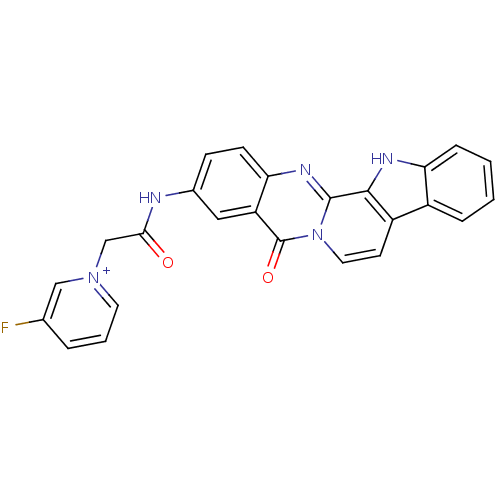 (CHEMBL2375941) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433028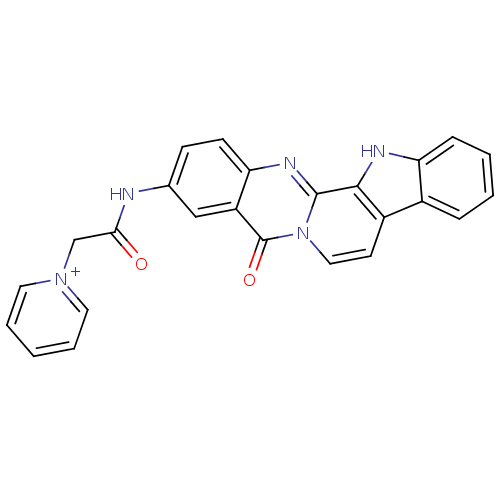 (CHEMBL2375940) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433018 (CHEMBL2375923) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433019 (CHEMBL2375922) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433022 (CHEMBL2375919) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433005 (CHEMBL2375936) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169042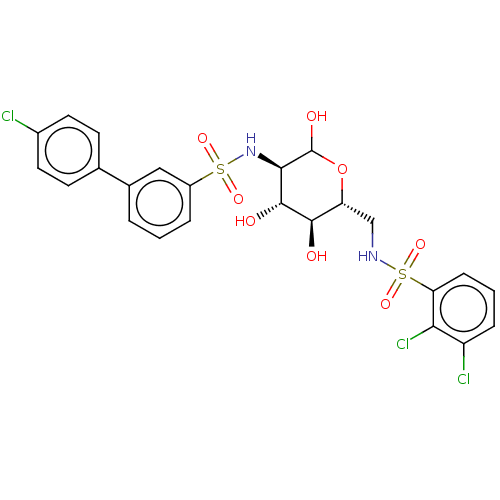 (CHEMBL3806103) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169033 (CHEMBL3806069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169038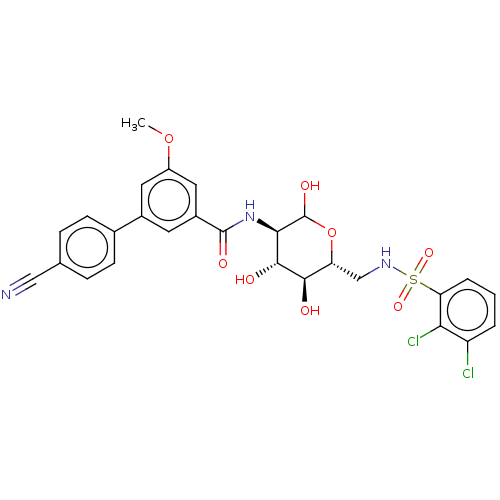 (CHEMBL3804841) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433026 (CHEMBL2375942) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433017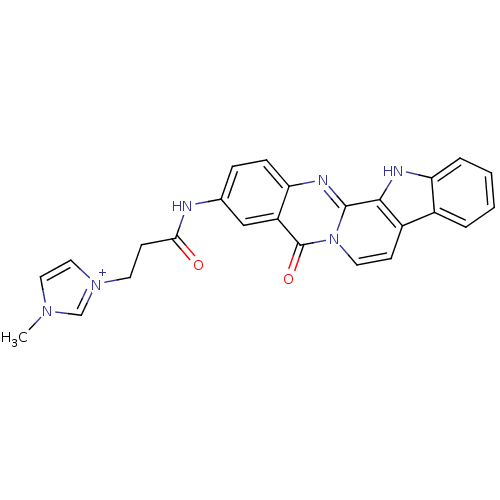 (CHEMBL2375924) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169037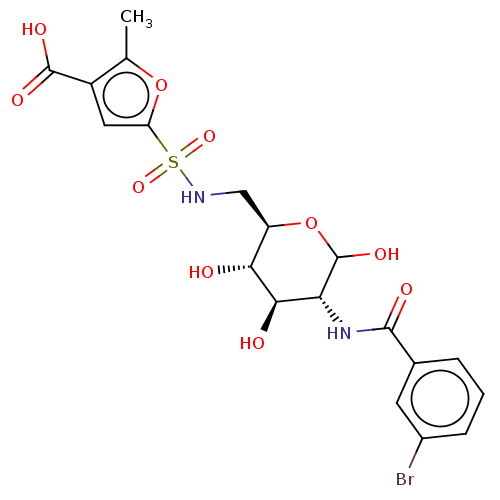 (CHEMBL3805205) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433004 (CHEMBL2375937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433014 (CHEMBL2375927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylthiocholine chloride as substrate preincubated for 15 mins before substrate addition by... | Bioorg Med Chem 20: 2527-34 (2012) Article DOI: 10.1016/j.bmc.2012.02.061 BindingDB Entry DOI: 10.7270/Q26111B3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433010 (CHEMBL2375931) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169026 (CHEMBL3805148) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169032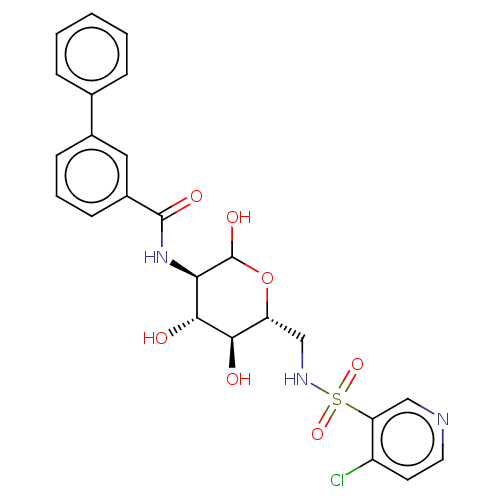 (CHEMBL3805398) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01058 BindingDB Entry DOI: 10.7270/Q2ZC86W8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169037 (CHEMBL3805205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50380184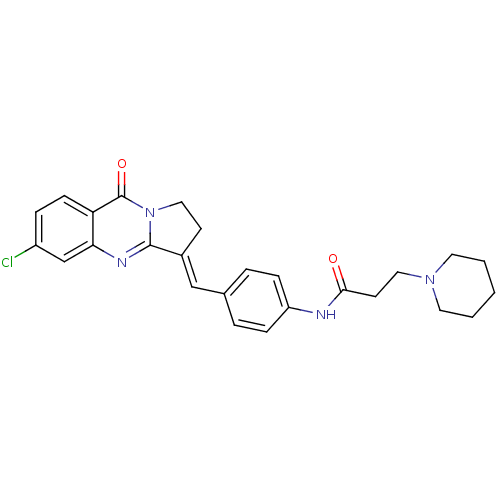 (CHEMBL2011200) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylcholine chloride as substrate preincubated for 15 mins before substrate addition by Ellma... | Bioorg Med Chem 20: 2527-34 (2012) Article DOI: 10.1016/j.bmc.2012.02.061 BindingDB Entry DOI: 10.7270/Q26111B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169041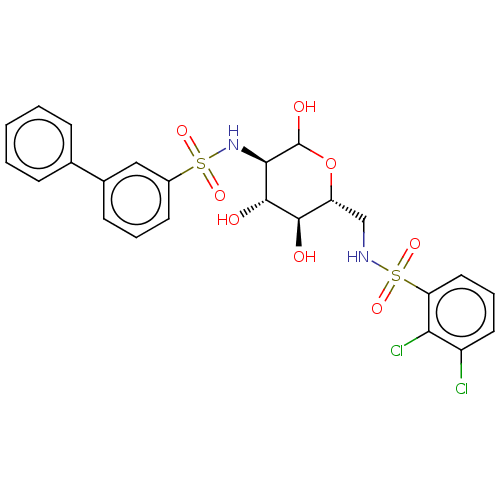 (CHEMBL3805653) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169034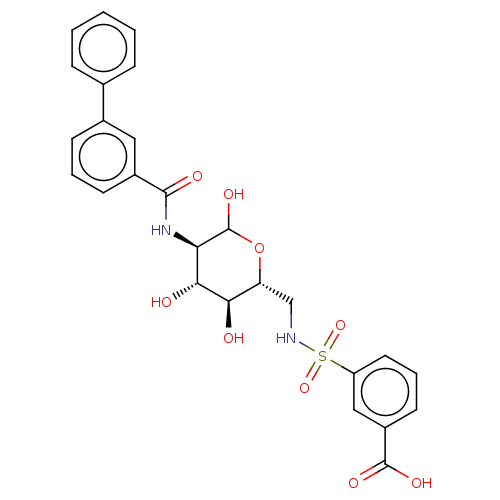 (CHEMBL3805734) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169031 (CHEMBL3805905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169038 (CHEMBL3804841) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50592935 (CHEMBL5192285) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01058 BindingDB Entry DOI: 10.7270/Q2ZC86W8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50380183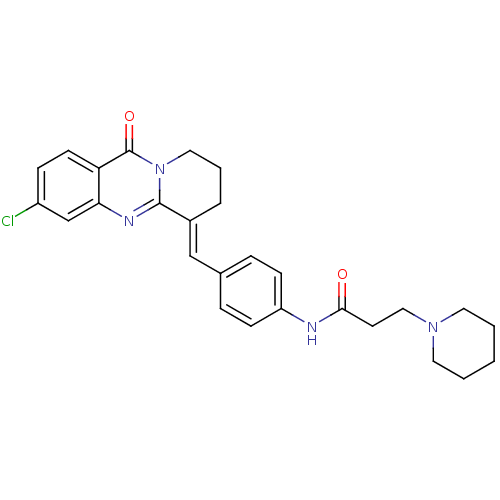 (CHEMBL2011201) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylcholine chloride as substrate preincubated for 15 mins before substrate addition by Ellma... | Bioorg Med Chem 20: 2527-34 (2012) Article DOI: 10.1016/j.bmc.2012.02.061 BindingDB Entry DOI: 10.7270/Q26111B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169031 (CHEMBL3805905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butylthiocholine as substrate incubated for 15 mins followed by substrate addition measured for... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169039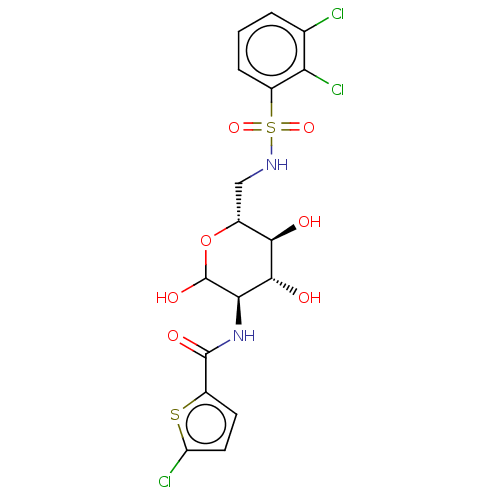 (CHEMBL3806132) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169017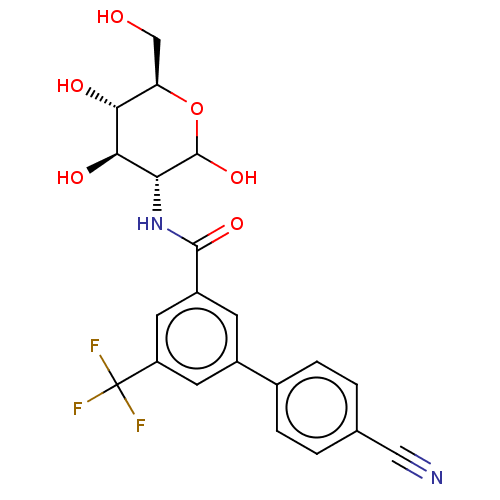 (CHEMBL3804930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50380187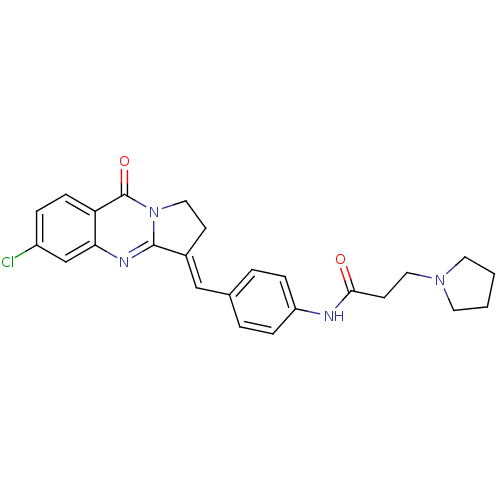 (CHEMBL2011196) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylcholine chloride as substrate preincubated for 15 mins before substrate addition by Ellma... | Bioorg Med Chem 20: 2527-34 (2012) Article DOI: 10.1016/j.bmc.2012.02.061 BindingDB Entry DOI: 10.7270/Q26111B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50380186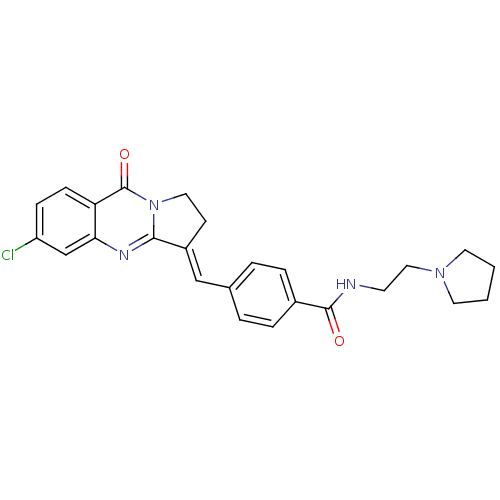 (CHEMBL2011202) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylcholine chloride as substrate preincubated for 15 mins before substrate addition by Ellma... | Bioorg Med Chem 20: 2527-34 (2012) Article DOI: 10.1016/j.bmc.2012.02.061 BindingDB Entry DOI: 10.7270/Q26111B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50380185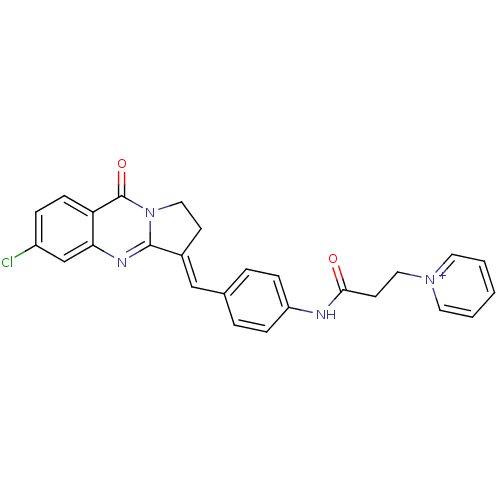 (CHEMBL2011194) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylcholine chloride as substrate preincubated for 15 mins before substrate addition by Ellma... | Bioorg Med Chem 20: 2527-34 (2012) Article DOI: 10.1016/j.bmc.2012.02.061 BindingDB Entry DOI: 10.7270/Q26111B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169043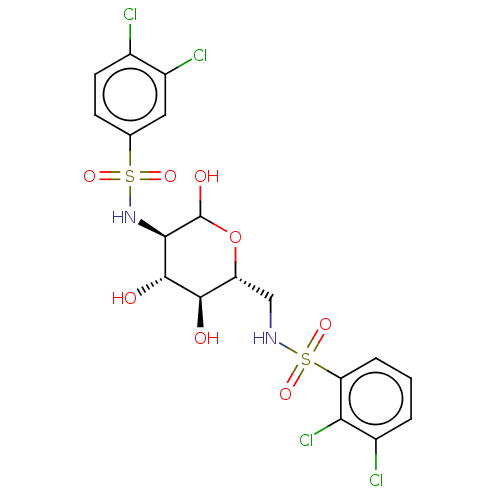 (CHEMBL3806095) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50592937 (CHEMBL5195044) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01058 BindingDB Entry DOI: 10.7270/Q2ZC86W8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169034 (CHEMBL3805734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433013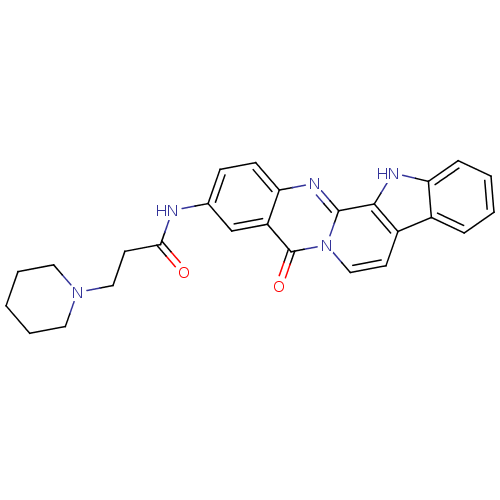 (CHEMBL2375928) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433016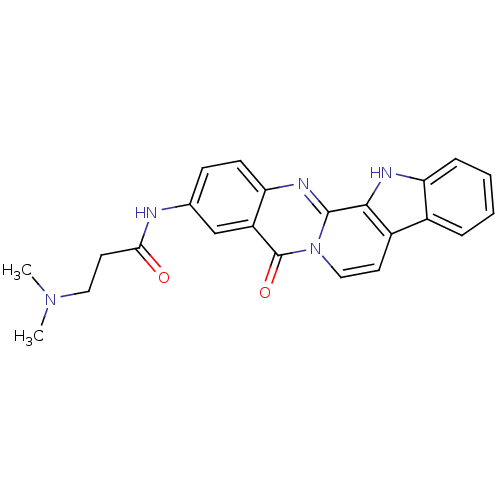 (CHEMBL2375925) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316368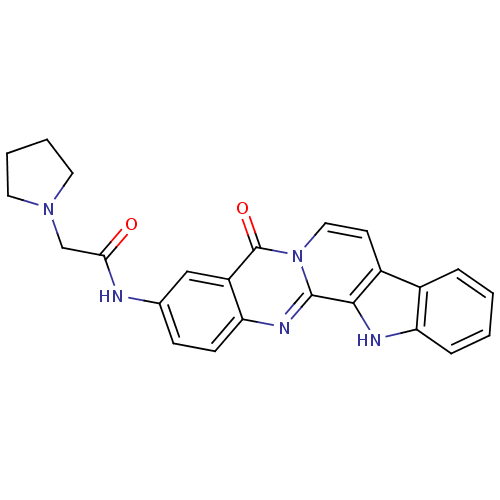 (3-(2-N-Pyrrolyl-acetamino)-7,8-dehydrorutaecarpine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50380182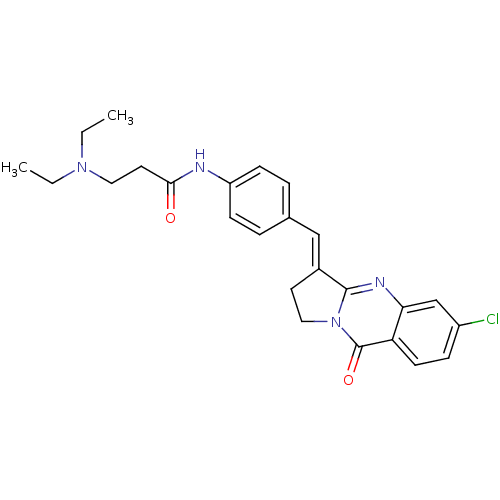 (CHEMBL2011198) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylcholine chloride as substrate preincubated for 15 mins before substrate addition by Ellma... | Bioorg Med Chem 20: 2527-34 (2012) Article DOI: 10.1016/j.bmc.2012.02.061 BindingDB Entry DOI: 10.7270/Q26111B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50592936 (CHEMBL5177837) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01058 BindingDB Entry DOI: 10.7270/Q2ZC86W8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433015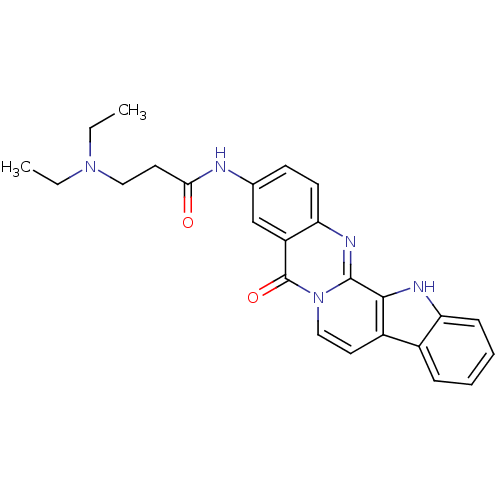 (CHEMBL2375926) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01058 BindingDB Entry DOI: 10.7270/Q2ZC86W8 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50592931 (CHEMBL5194009) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01058 BindingDB Entry DOI: 10.7270/Q2ZC86W8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316369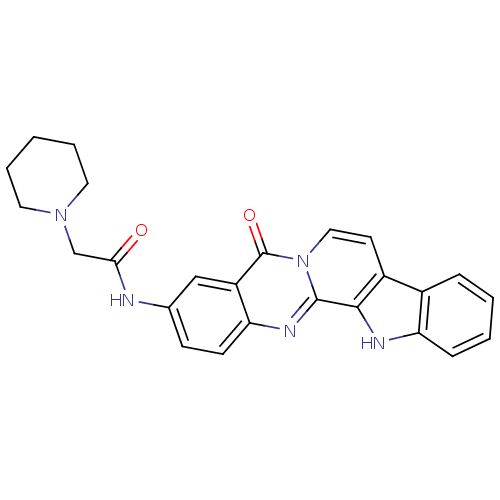 (3-(2-N-Piperidyl-acetamino)-7,8-dehydrorutaecarpin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316367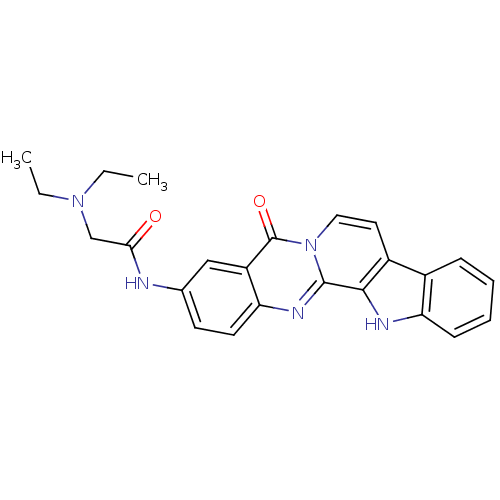 (3-(2-Diethylamino-acetamino)-7,8-dehydrorutaecarpi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 311 total ) | Next | Last >> |