

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078163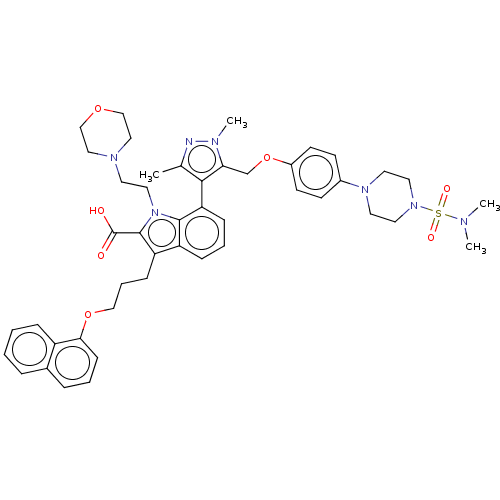 (CHEMBL3417704) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365773 (CHEMBL1956552) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Non-competitive inhibition of Electrophorus electricus AChE assessed as hydrolysis of acetylthiocholineiodide after 15 mins incubation by spectrophot... | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560862 (CHEMBL4796830) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560861 (CHEMBL4757050) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560860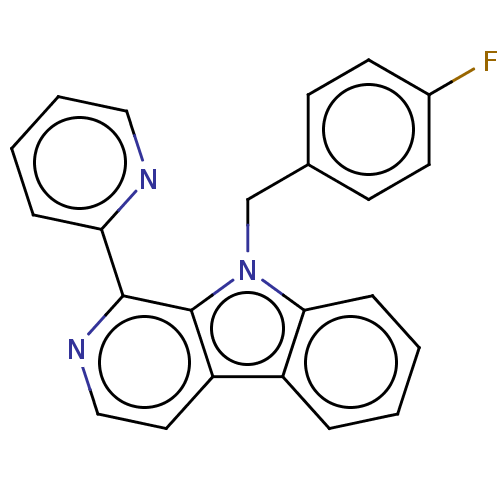 (CHEMBL4758555) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560859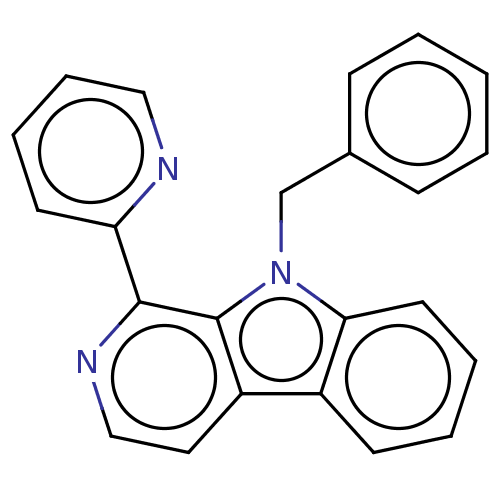 (CHEMBL4744295) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560865 (CHEMBL4778579) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560867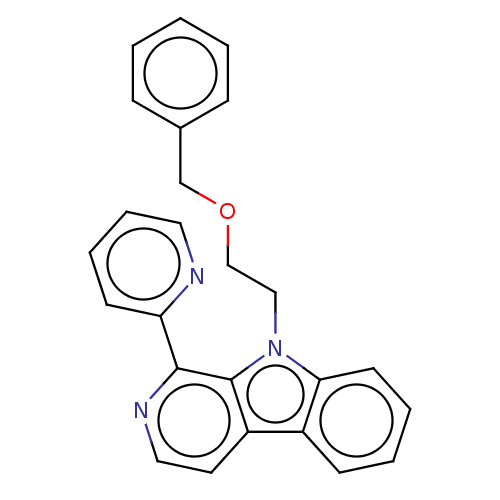 (CHEMBL4794980) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560866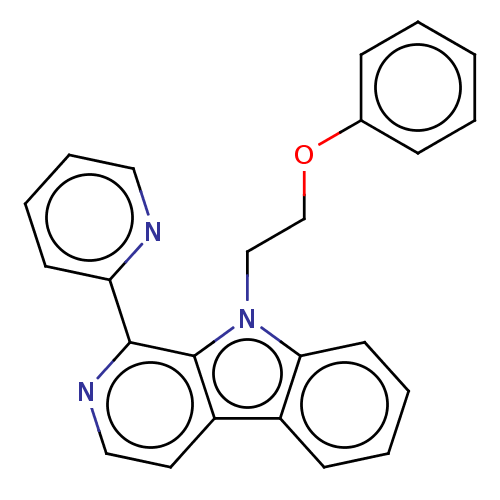 (CHEMBL4760810) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560864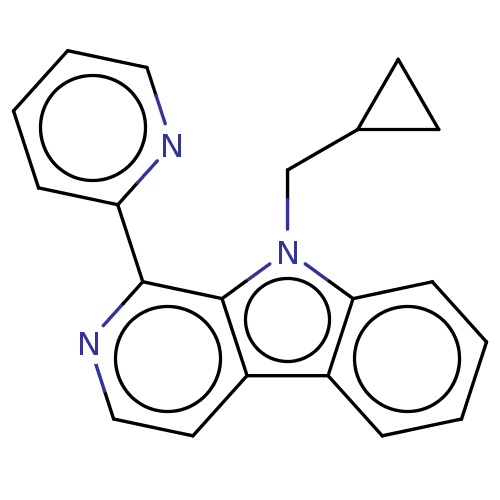 (CHEMBL4777267) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560858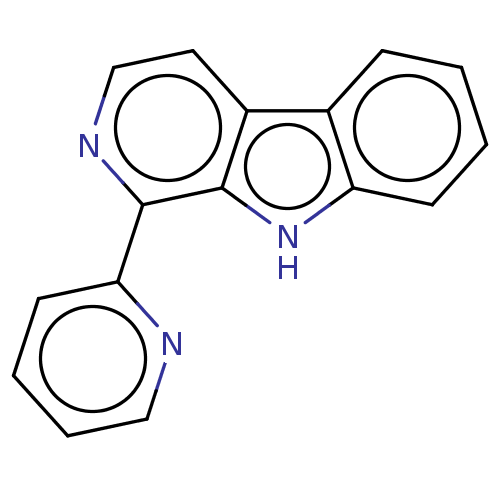 (CHEMBL4578154) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560863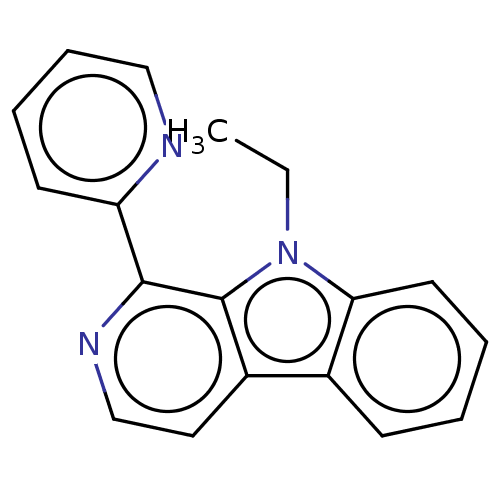 (CHEMBL4743955) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50560868 (CHEMBL4748351) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353684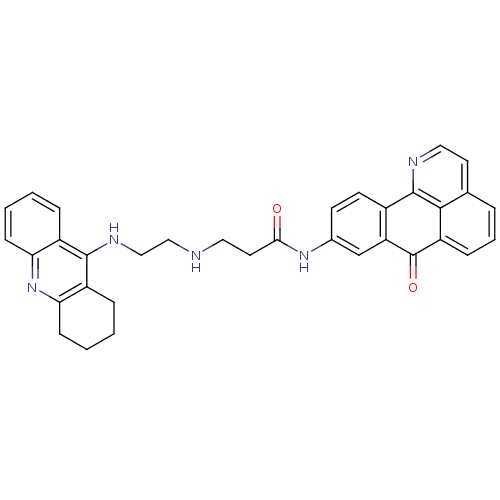 (CHEMBL1830627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078163 (CHEMBL3417704) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal biotin-labeled BIM BH3 peptide (141 to 166 residues) binding to His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078163 (CHEMBL3417704) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of biotinylated Bim peptide (81 to 106 residues) binding to His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherich... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02047 BindingDB Entry DOI: 10.7270/Q22J6GKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353680 (CHEMBL1830632) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353685 (CHEMBL1830626) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353681 (CHEMBL1830631) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353683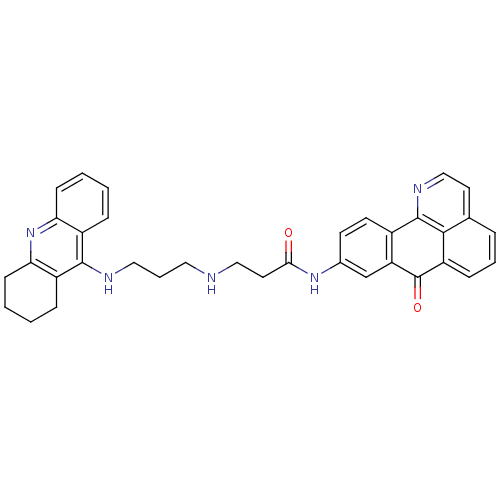 (CHEMBL1830628) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353689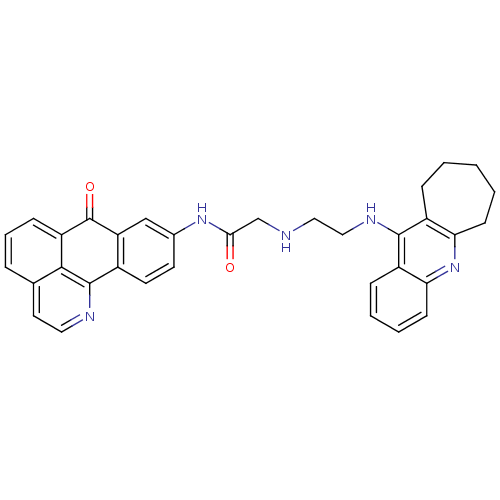 (CHEMBL1830630) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353681 (CHEMBL1830631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353684 (CHEMBL1830627) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353679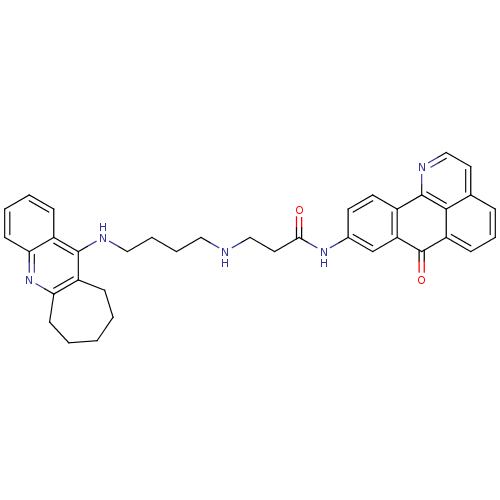 (CHEMBL1830633) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353682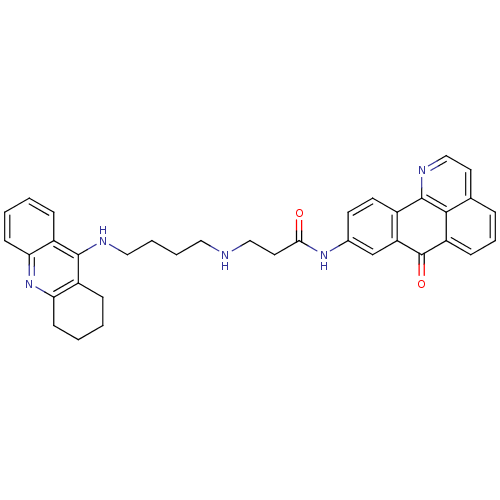 (CHEMBL1830629) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353683 (CHEMBL1830628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353686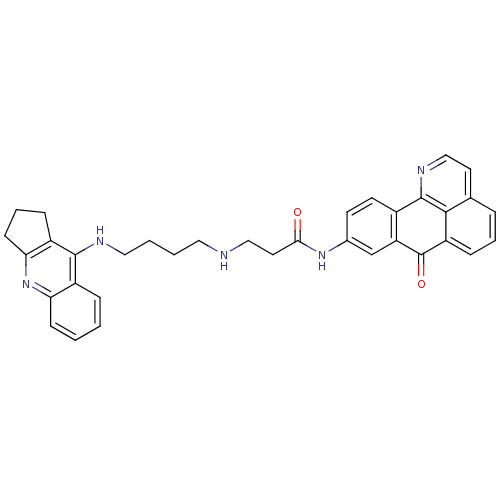 (CHEMBL1830625) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365773 (CHEMBL1956552) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353689 (CHEMBL1830630) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353688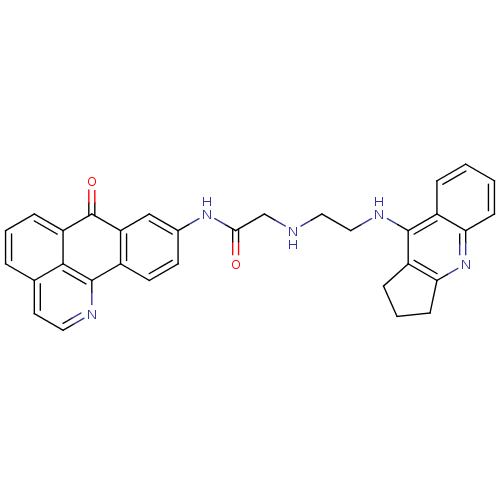 (CHEMBL1830622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353680 (CHEMBL1830632) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353682 (CHEMBL1830629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353687 (CHEMBL1830624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 399 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353687 (CHEMBL1830624) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353690 (CHEMBL1830623) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353685 (CHEMBL1830626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365771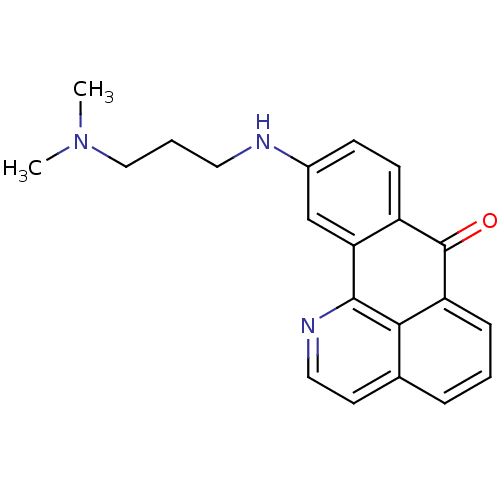 (CHEMBL1956550) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353688 (CHEMBL1830622) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365781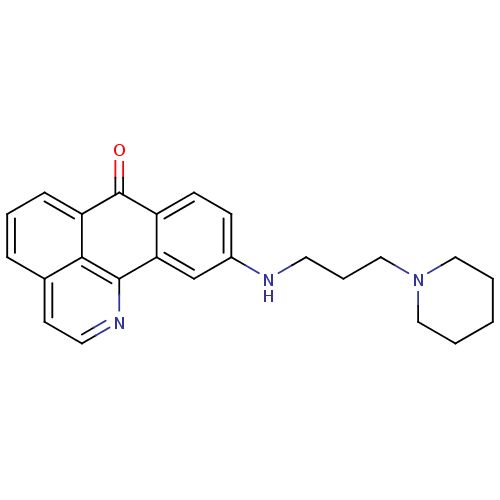 (CHEMBL1956560) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365772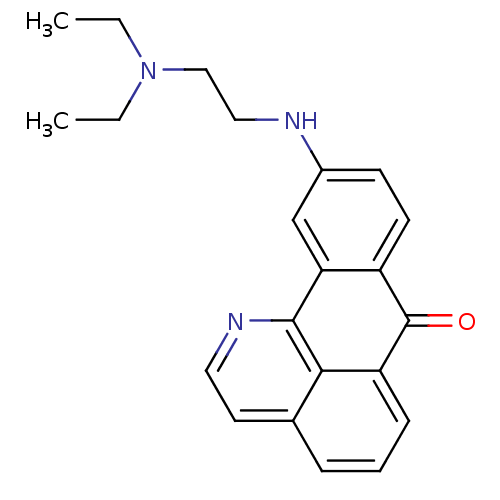 (CHEMBL1956551) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365779 (CHEMBL1956558) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353679 (CHEMBL1830633) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 745 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353686 (CHEMBL1830625) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365780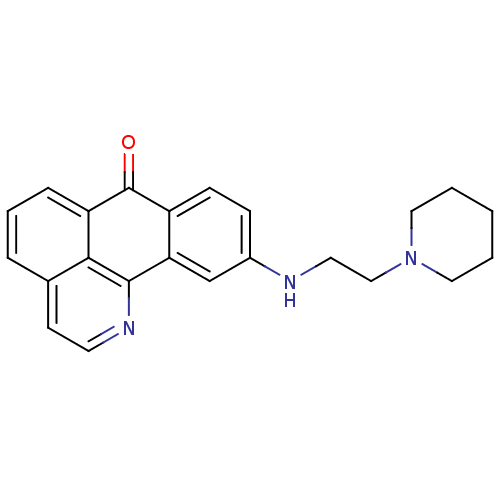 (CHEMBL1956559) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365772 (CHEMBL1956551) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365771 (CHEMBL1956550) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |