 Found 3350 hits with Last Name = 'cheung' and Initial = 'm'
Found 3350 hits with Last Name = 'cheung' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase TNNI3K
(Homo sapiens (Human)) | BDBM50578225
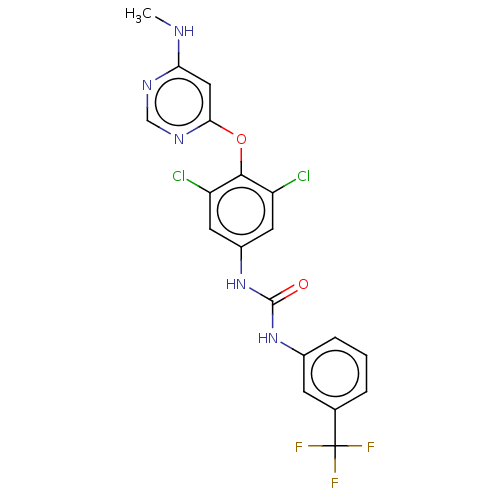
(CHEMBL4869303)Show SMILES CNc1cc(Oc2c(Cl)cc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2Cl)ncn1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length human His-MBP-TNNI3K assessed as off-rate constant in presence of rhodamine green labeled GW805818X by fluorescence c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin receptor
(Homo sapiens (Human)) | BDBM50256480
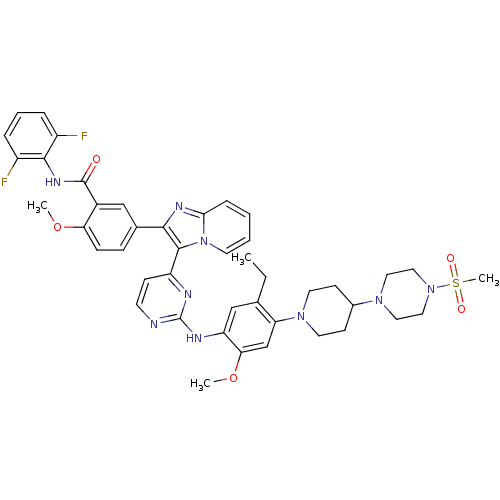
(CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...)Show SMILES CCc1cc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2ccc(OC)c(c2)C(=O)Nc2c(F)cccc2F)c(OC)cc1N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C44H47F2N9O5S/c1-5-28-26-35(38(60-3)27-36(28)53-19-15-30(16-20-53)52-21-23-54(24-22-52)61(4,57)58)49-44-47-17-14-34(48-44)42-40(50-39-11-6-7-18-55(39)42)29-12-13-37(59-2)31(25-29)43(56)51-41-32(45)9-8-10-33(41)46/h6-14,17-18,25-27,30H,5,15-16,19-24H2,1-4H3,(H,51,56)(H,47,48,49) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to insulin receptor by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256480

(CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...)Show SMILES CCc1cc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2ccc(OC)c(c2)C(=O)Nc2c(F)cccc2F)c(OC)cc1N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C44H47F2N9O5S/c1-5-28-26-35(38(60-3)27-36(28)53-19-15-30(16-20-53)52-21-23-54(24-22-52)61(4,57)58)49-44-47-17-14-34(48-44)42-40(50-39-11-6-7-18-55(39)42)29-12-13-37(59-2)31(25-29)43(56)51-41-32(45)9-8-10-33(41)46/h6-14,17-18,25-27,30H,5,15-16,19-24H2,1-4H3,(H,51,56)(H,47,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to IGF1R by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50256478
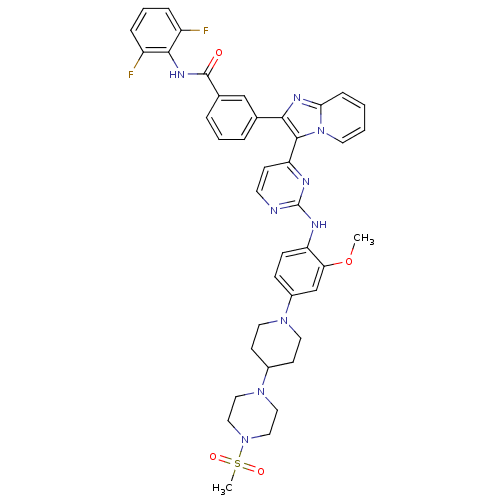
(CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C41H41F2N9O4S/c1-56-35-26-30(49-19-15-29(16-20-49)50-21-23-51(24-22-50)57(2,54)55)12-13-33(35)45-41-44-17-14-34(46-41)39-37(47-36-11-3-4-18-52(36)39)27-7-5-8-28(25-27)40(53)48-38-31(42)9-6-10-32(38)43/h3-14,17-18,25-26,29H,15-16,19-24H2,1-2H3,(H,48,53)(H,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to insulin receptor by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256478

(CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C41H41F2N9O4S/c1-56-35-26-30(49-19-15-29(16-20-49)50-21-23-51(24-22-50)57(2,54)55)12-13-33(35)45-41-44-17-14-34(46-41)39-37(47-36-11-3-4-18-52(36)39)27-7-5-8-28(25-27)40(53)48-38-31(42)9-6-10-32(38)43/h3-14,17-18,25-26,29H,15-16,19-24H2,1-2H3,(H,48,53)(H,44,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to IGF1R by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162995
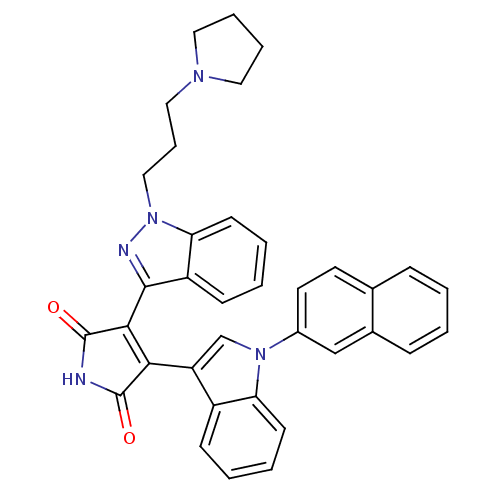
(3-[1-(1,2-Dihydro-pyridin-2-yl)-1H-indazol-3-yl]-4...)Show SMILES O=C1NC(=O)C(=C1c1cn(-c2ccc3ccccc3c2)c2ccccc12)c1nn(CCCN2CCCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C36H31N5O2/c42-35-32(29-23-40(30-14-5-3-12-27(29)30)26-17-16-24-10-1-2-11-25(24)22-26)33(36(43)37-35)34-28-13-4-6-15-31(28)41(38-34)21-9-20-39-18-7-8-19-39/h1-6,10-17,22-23H,7-9,18-21H2,(H,37,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TNNI3K
(Mus musculus) | BDBM50578225

(CHEMBL4869303)Show SMILES CNc1cc(Oc2c(Cl)cc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2Cl)ncn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length mouse His-MBP-TNNI3K assessed as off-rate constant in presence of rhodamine green labeled GW805818X by fluorescence c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162996
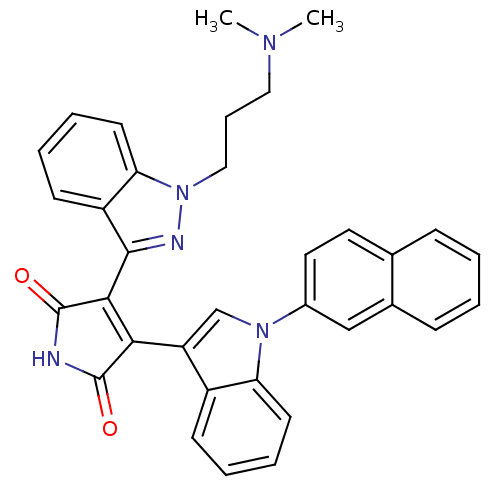
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162990
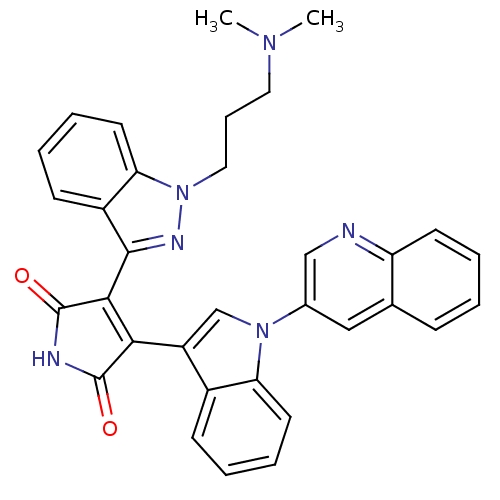
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50163000
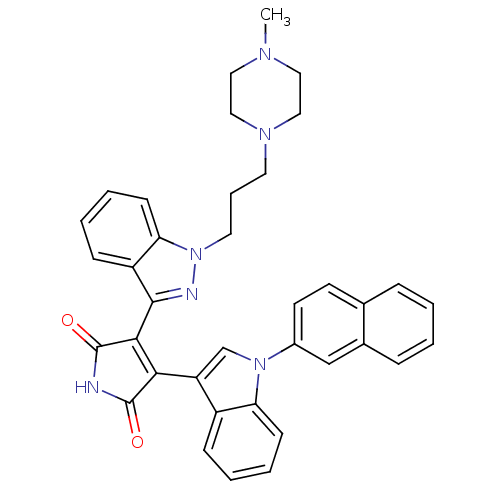
(3-(1-Piperazin-1-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES CN1CCN(CCCn2nc(C3=C(C(=O)NC3=O)c3cn(-c4ccc5ccccc5c4)c4ccccc34)c3ccccc23)CC1 |t:11| Show InChI InChI=1S/C37H34N6O2/c1-40-19-21-41(22-20-40)17-8-18-43-32-14-7-5-12-29(32)35(39-43)34-33(36(44)38-37(34)45)30-24-42(31-13-6-4-11-28(30)31)27-16-15-25-9-2-3-10-26(25)23-27/h2-7,9-16,23-24H,8,17-22H2,1H3,(H,38,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50017376

((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162992
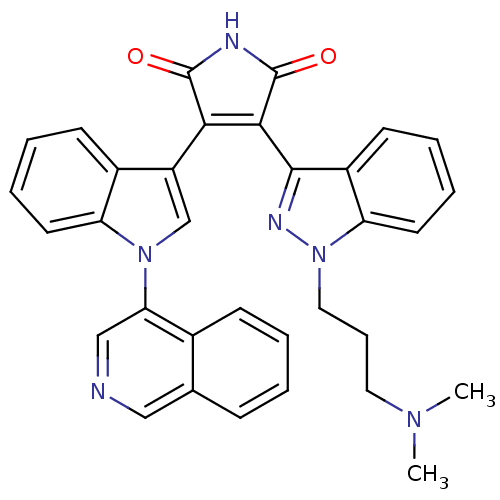
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cncc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-27-15-8-6-13-24(27)31(36-39)30-29(32(40)35-33(30)41)25-20-38(26-14-7-5-12-23(25)26)28-19-34-18-21-10-3-4-11-22(21)28/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50162993
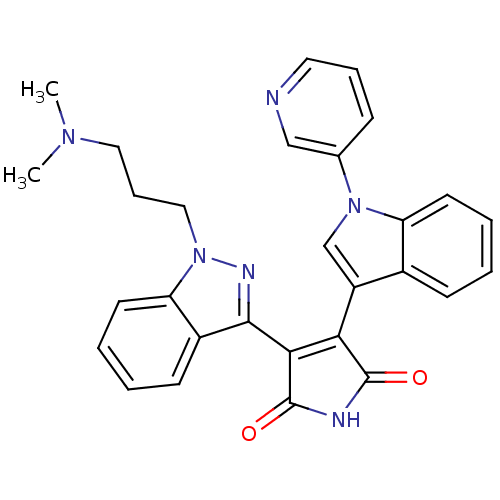
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccnc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H26N6O2/c1-33(2)15-8-16-35-24-13-6-4-11-21(24)27(32-35)26-25(28(36)31-29(26)37)22-18-34(19-9-7-14-30-17-19)23-12-5-3-10-20(22)23/h3-7,9-14,17-18H,8,15-16H2,1-2H3,(H,31,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50163002
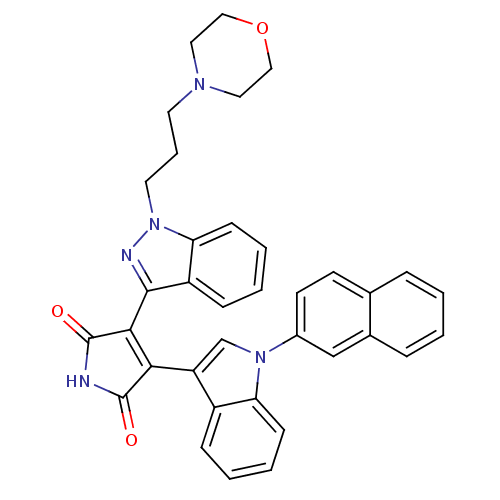
(3-(1-Morpholin-2-yl-1H-indazol-3-yl)-4-[1-(1,4,4a,...)Show SMILES O=C1NC(=O)C(=C1c1cn(-c2ccc3ccccc3c2)c2ccccc12)c1nn(CCCN2CCOCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C36H31N5O3/c42-35-32(29-23-40(30-12-5-3-10-27(29)30)26-15-14-24-8-1-2-9-25(24)22-26)33(36(43)37-35)34-28-11-4-6-13-31(28)41(38-34)17-7-16-39-18-20-44-21-19-39/h1-6,8-15,22-23H,7,16-21H2,(H,37,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Potassium channel HERG |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621456

(US11773078, Example 208)Show SMILES CNC(=O)OCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CC3)C(C)CCC(O)=O)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621311

(US11773078, Example 63)Show SMILES COC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(C)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621405

(US11773078, Example 157)Show SMILES CN1CCN(CC1)c1ccc(Oc2cc(CN3CCC(CNC(C)=O)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621419

(US11773078, Example 171)Show SMILES CNC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CCCO)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621438
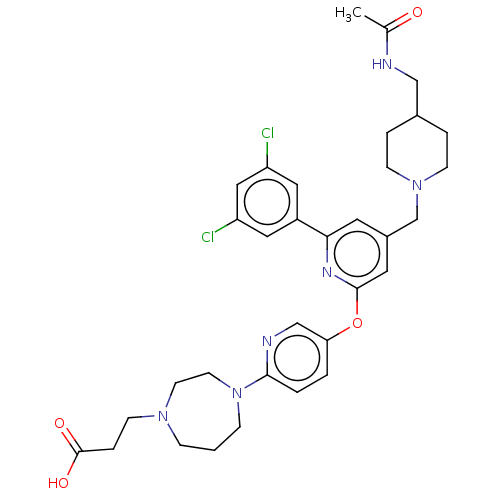
(US11773078, Example 190)Show SMILES CC(=O)NCC1CCN(Cc2cc(Oc3ccc(nc3)N3CCCN(CCC(O)=O)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621539

(US11773078, Example 291)Show SMILES CNC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CC(C)C(N)=O)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621266
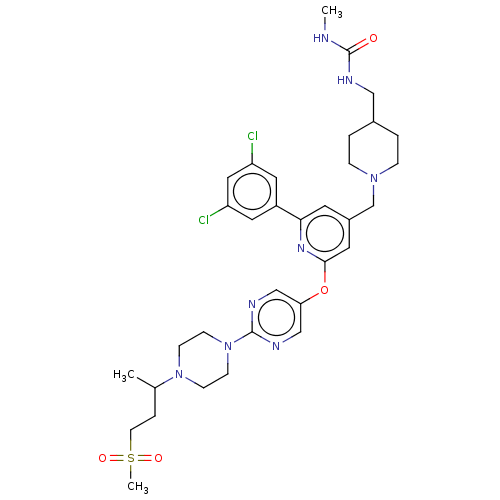
(US11773078, Example 18)Show SMILES CNC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CC3)C(C)CCS(C)(=O)=O)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621482

(US11773078, Example 234)Show SMILES CCN1CCN(CC1)c1ncc(Oc2cc(CN3CCC(CC(O)=O)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621431
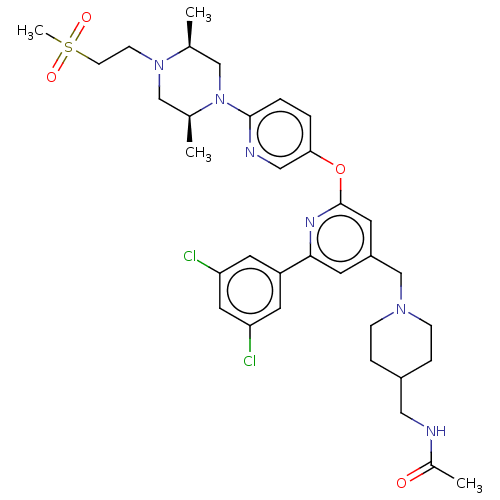
(US11773078, Example 183)Show SMILES C[C@H]1CN([C@@H](C)CN1CCS(C)(=O)=O)c1ccc(Oc2cc(CN3CCC(CNC(C)=O)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)cn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621544

(US11773078, Example 296)Show SMILES CC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CC4CCC(C3)N4CCC(O)=O)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621610

(US11773078, Example 362)Show SMILES CN1CCCN(CC1)c1ccc(Oc2cc(CN3CC[C@H]4[C@@H](CC3)[C@@H]4C(O)=O)cc(n2)-c2cc(Cl)cc(Cl)c2)nn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621496

(US11773078, Example 248)Show SMILES CNC(=O)OCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CCC(O)=O)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621525
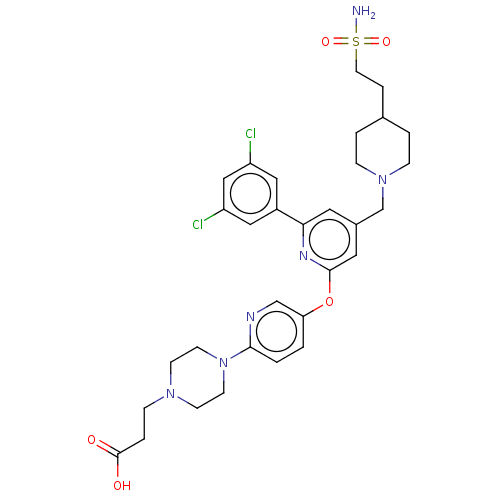
(US11773078, Example 277)Show SMILES NS(=O)(=O)CCC1CCN(Cc2cc(Oc3ccc(nc3)N3CCN(CCC(O)=O)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621265
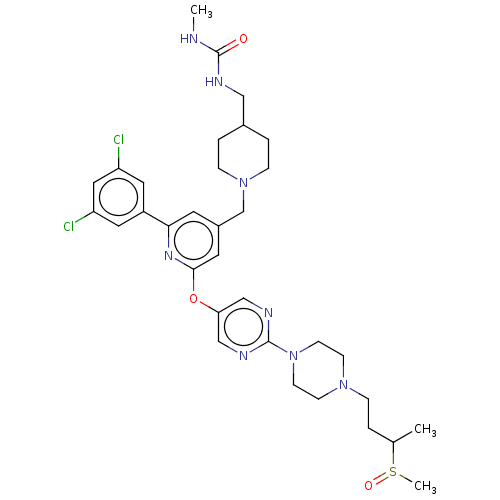
(US11773078, Example 17)Show SMILES CNC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CCC(C)S(C)=O)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621304

(US11773078, Example 56)Show SMILES COC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CC4CCC(C3)N4CCC(O)=O)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621318

(US11773078, Example 70)Show SMILES CNC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(C)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621417
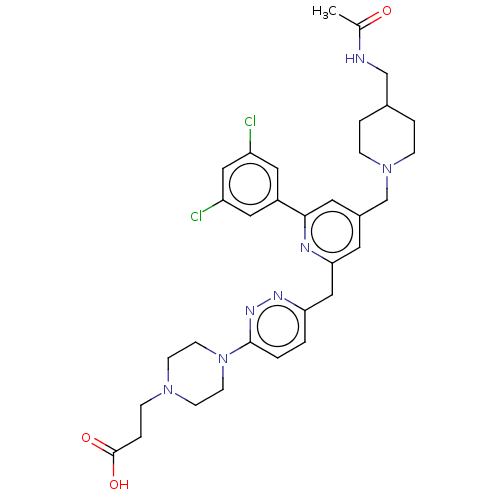
(US11773078, Example 169)Show SMILES CC(=O)NCC1CCN(Cc2cc(Cc3ccc(nn3)N3CCN(CCC(O)=O)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621440
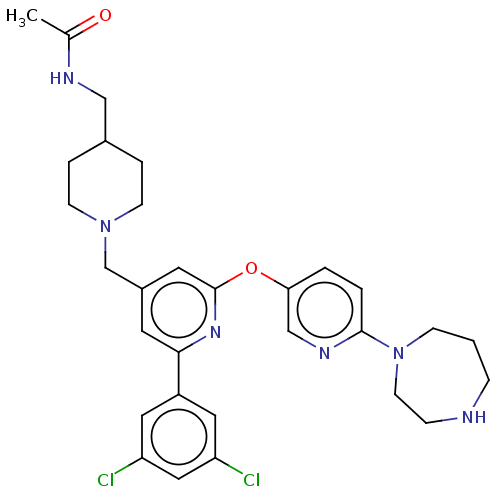
(US11773078, Example 192)Show SMILES CC(=O)NCC1CCN(Cc2cc(Oc3ccc(nc3)N3CCCNCC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621314

(US11773078, Example 66 | US11773078, Example 67)Show SMILES CNC(=O)OCC1CCN(Cc2cc(Oc3ccc(nc3)N3CCN(C)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621364

(US11773078, Example 116)Show SMILES CC(=O)NCC1CCN(Cc2cc(Oc3ccc(nc3)N3CCN(CCC(O)=O)CC3)nc(c2)-c2cc(F)cc(Br)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621469

(US11773078, Example 221)Show SMILES CNC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CC3)C3CC(C3)C(O)=O)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 |(14.95,-1.31,;13.61,-2.08,;12.28,-1.31,;12.28,.23,;10.95,-2.08,;9.61,-1.31,;8.28,-2.08,;8.28,-3.62,;6.94,-4.39,;5.61,-3.62,;4.28,-4.39,;2.94,-3.62,;1.61,-4.39,;.28,-3.62,;-1.06,-4.39,;-2.39,-3.62,;-3.72,-4.39,;-5.06,-3.62,;-5.06,-2.08,;-3.72,-1.31,;-2.39,-2.08,;-6.39,-1.31,;-7.73,-2.08,;-9.06,-1.31,;-9.06,.23,;-7.73,1,;-6.39,.23,;-10.39,1,;-10.79,2.48,;-12.28,2.08,;-11.88,.6,;-13.61,2.85,;-14.95,2.08,;-13.61,4.39,;.28,-2.08,;1.61,-1.31,;2.94,-2.08,;1.61,.23,;.28,1,;.28,2.54,;-1.06,3.31,;1.61,3.31,;2.94,2.54,;4.28,3.31,;2.94,1,;5.61,-2.08,;6.94,-1.31,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621358

(US11773078, Example 110)Show SMILES CN1CCN(CC1)c1cnc(Oc2cc(CN3CCC(CNC(C)=O)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621644
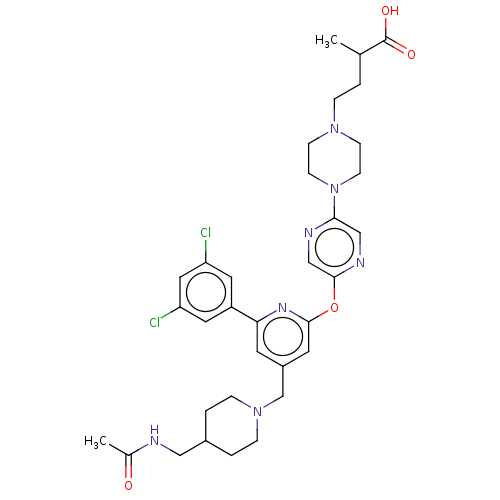
(US11773078, Example 396)Show SMILES CC(CCN1CCN(CC1)c1cnc(Oc2cc(CN3CCC(CNC(C)=O)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)cn1)C(O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621305

(US11773078, Example 57)Show SMILES CC(CCN1CCN(CC1)c1ncc(Oc2cc(CN3CCC(CCO)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)cn1)C(O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621464
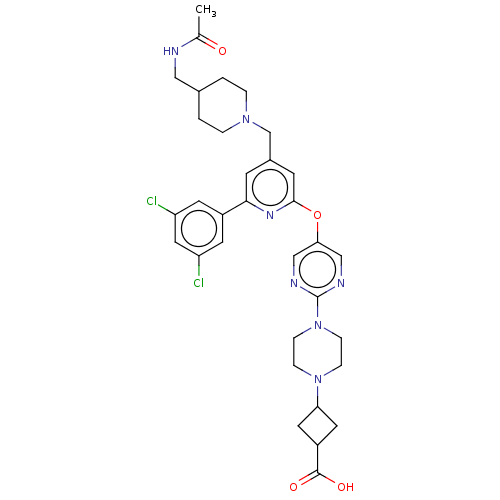
(US11773078, Example 216)Show SMILES CC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CC3)C3CC(C3)C(O)=O)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 |(14.28,-2.08,;12.95,-1.31,;12.95,.23,;11.61,-2.08,;10.28,-1.31,;8.94,-2.08,;8.94,-3.62,;7.61,-4.39,;6.28,-3.62,;4.94,-4.39,;3.61,-3.62,;2.28,-4.39,;.94,-3.62,;-.39,-4.39,;-1.72,-3.62,;-3.06,-4.39,;-4.39,-3.62,;-4.39,-2.08,;-3.06,-1.31,;-1.72,-2.08,;-5.73,-1.31,;-7.06,-2.08,;-8.39,-1.31,;-8.39,.23,;-7.06,1,;-5.73,.23,;-9.73,1,;-10.12,2.48,;-11.61,2.08,;-11.21,.6,;-12.95,2.85,;-14.28,2.08,;-12.95,4.39,;.94,-2.08,;2.28,-1.31,;3.61,-2.08,;2.28,.23,;.94,1,;.94,2.54,;-.39,3.31,;2.28,3.31,;3.61,2.54,;4.94,3.31,;3.61,1,;6.28,-2.08,;7.61,-1.31,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621564

(US11773078, Example 316)Show SMILES CC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CC(O)C(O)=O)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621267
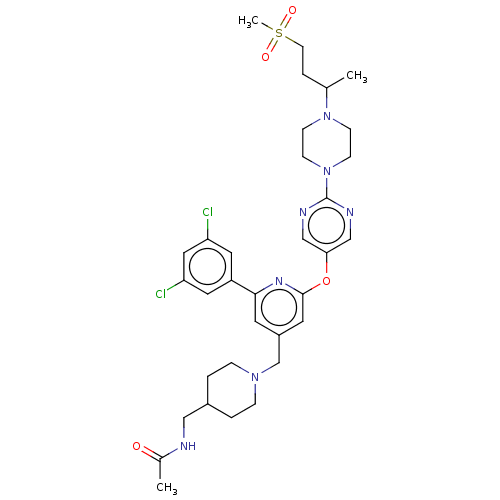
(US11773078, Example 19)Show SMILES CC(CCS(C)(=O)=O)N1CCN(CC1)c1ncc(Oc2cc(CN3CCC(CNC(C)=O)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621470

(US11773078, Example 222)Show SMILES COC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CC3)C(C)CCC(O)=O)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621468

(US11773078, Example 220)Show SMILES CNC(=O)NCC1CCN(Cc2cc(Cc3cnc(nc3)N3CCN(CCC(C)S(C)(=O)=O)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621459

(US11773078, Example 211)Show SMILES CNC(=O)OCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CC3)C3CC(C3)C(O)=O)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 |(14.95,-1.31,;13.61,-2.08,;12.28,-1.31,;12.28,.23,;10.95,-2.08,;9.61,-1.31,;8.28,-2.08,;8.28,-3.62,;6.94,-4.39,;5.61,-3.62,;4.28,-4.39,;2.94,-3.62,;1.61,-4.39,;.28,-3.62,;-1.06,-4.39,;-2.39,-3.62,;-3.72,-4.39,;-5.06,-3.62,;-5.06,-2.08,;-3.72,-1.31,;-2.39,-2.08,;-6.39,-1.31,;-7.73,-2.08,;-9.06,-1.31,;-9.06,.23,;-7.73,1,;-6.39,.23,;-10.39,1,;-10.79,2.48,;-12.28,2.08,;-11.88,.6,;-13.61,2.85,;-14.95,2.08,;-13.61,4.39,;.28,-2.08,;1.61,-1.31,;2.94,-2.08,;1.61,.23,;.28,1,;.28,2.54,;-1.06,3.31,;1.61,3.31,;2.94,2.54,;4.28,3.31,;2.94,1,;5.61,-2.08,;6.94,-1.31,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621466

(US11773078, Example 218)Show SMILES CC(CCN1CCN(CC1)c1ccc(Oc2cc(CN3CCC(CNC(C)=O)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)cn1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621532
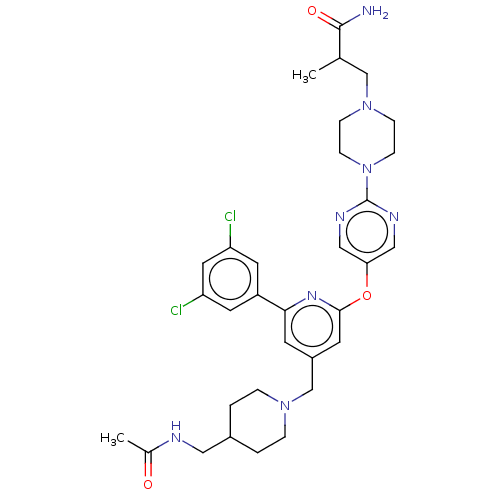
(US11773078, Example 284)Show SMILES CC(CN1CCN(CC1)c1ncc(Oc2cc(CN3CCC(CNC(C)=O)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)cn1)C(N)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621615
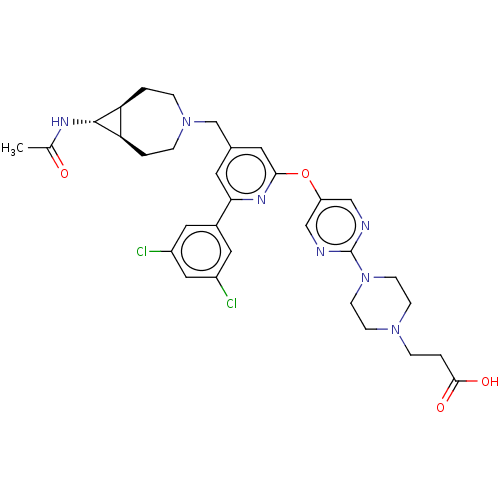
(US11773078, Example 367)Show SMILES CC(=O)N[C@@H]1[C@H]2CCN(Cc3cc(Oc4cnc(nc4)N4CCN(CCC(O)=O)CC4)nc(c3)-c3cc(Cl)cc(Cl)c3)CC[C@@H]12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621505
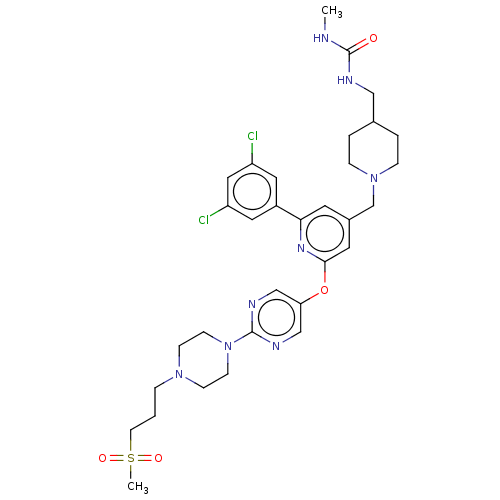
(US11773078, Example 257)Show SMILES CNC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CCCS(C)(=O)=O)CC3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621542
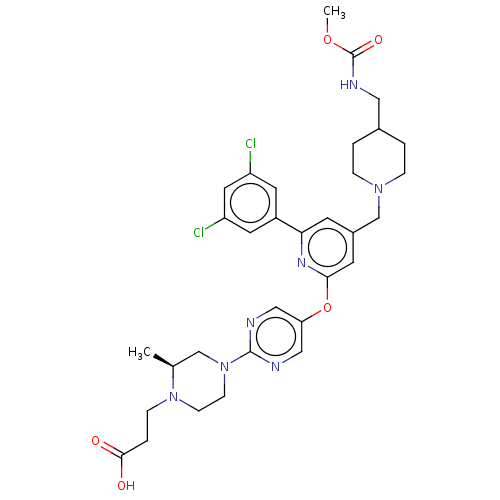
(US11773078, Example 294)Show SMILES COC(=O)NCC1CCN(Cc2cc(Oc3cnc(nc3)N3CCN(CCC(O)=O)[C@@H](C)C3)nc(c2)-c2cc(Cl)cc(Cl)c2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Furin [108-574]
(Homo sapiens (Human)) | BDBM621493
(US11773078, Example 245)Show SMILES COCCN1CCN(CC1)c1ncc(Oc2cc(CN3CCC(CNC(C)=O)CC3)cc(n2)-c2cc(Cl)cc(Cl)c2)cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data