

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198754 (CHEMBL3924888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198760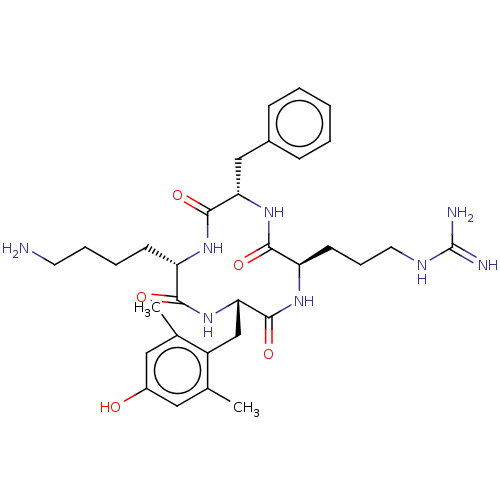 (CHEMBL3897031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198758 (CHEMBL3908315) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0745 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001157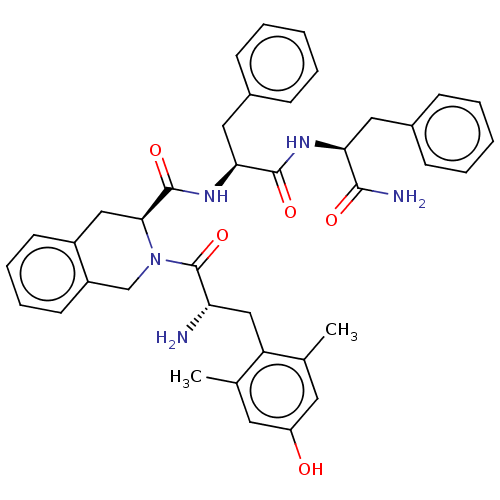 (CHEMBL538700) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198755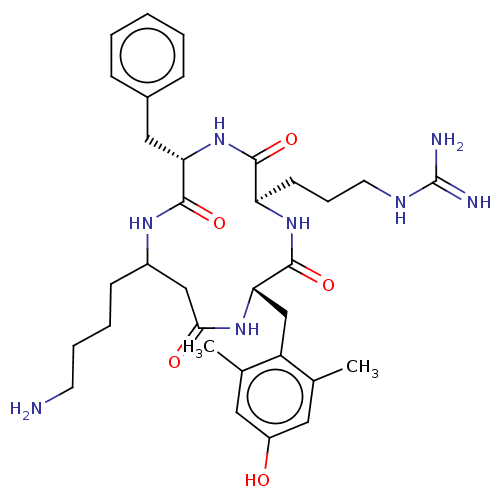 (CHEMBL3979449) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198757 (CHEMBL363142) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080453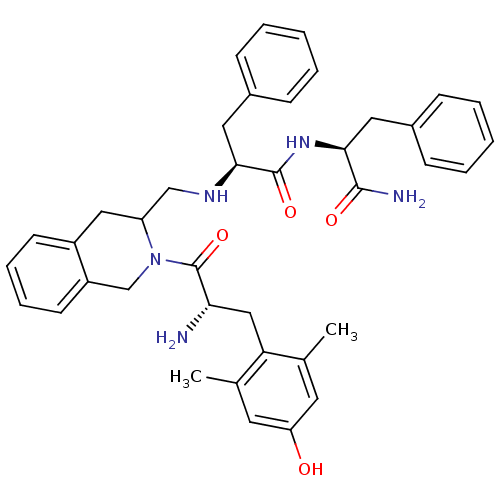 ((S)-2-({2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198754 (CHEMBL3924888) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.733 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198755 (CHEMBL3979449) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.786 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198754 (CHEMBL3924888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.807 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080453 ((S)-2-({2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description . Binding affinity for Opioid receptor mu 1 determined by displacing [3H]-DAMGO from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198752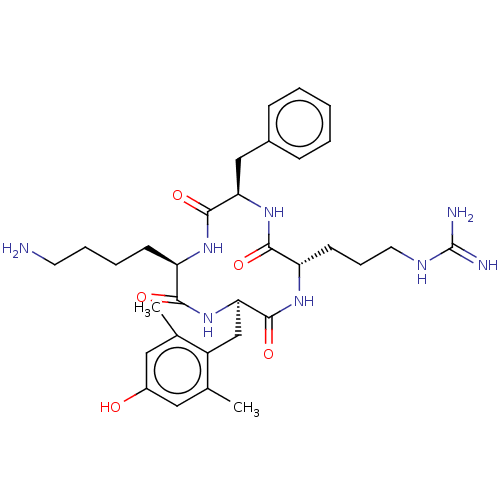 (CHEMBL3976694) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001157 (CHEMBL538700) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description . Binding affinity for Opioid receptor mu 1 determined by displacing [3H]-DAMGO from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50252861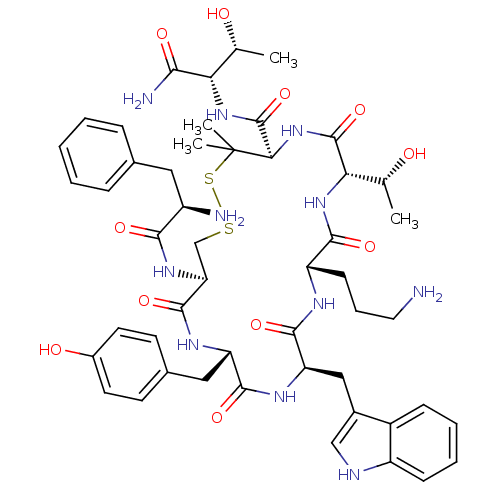 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198760 (CHEMBL3897031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198756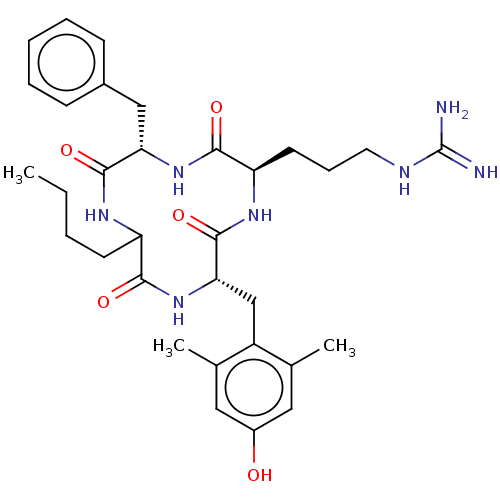 (CHEMBL3951723) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080452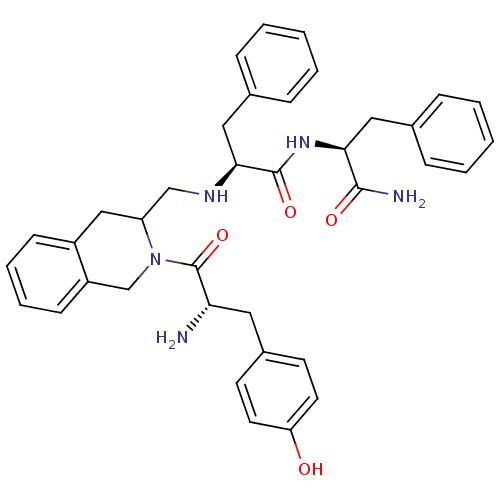 ((S)-2-({2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198758 (CHEMBL3908315) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080454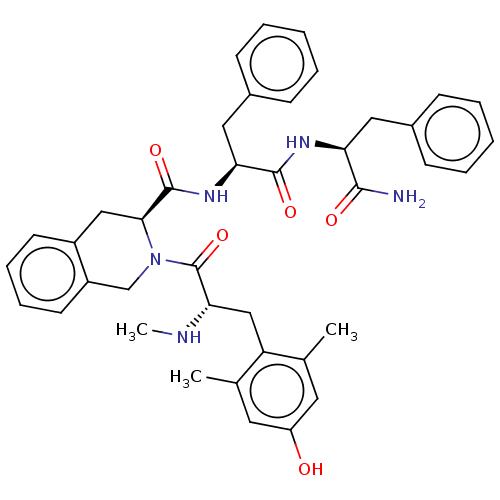 (2-[(S)-3-(4-Hydroxy-2,6-dimethyl-phenyl)-2-methyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080454 (2-[(S)-3-(4-Hydroxy-2,6-dimethyl-phenyl)-2-methyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description . Binding affinity for Opioid receptor mu 1 determined by displacing [3H]-DAMGO from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198758 (CHEMBL3908315) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198760 (CHEMBL3897031) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198753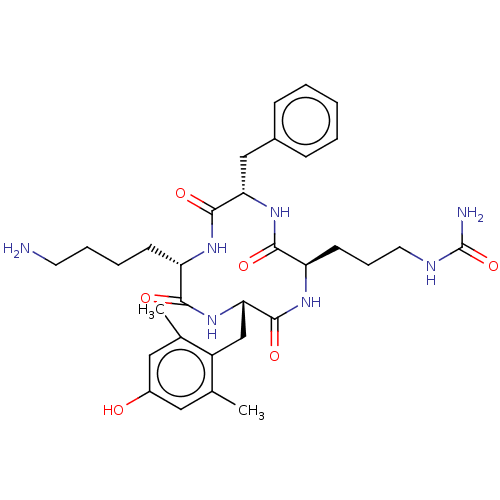 (CHEMBL3944636) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198759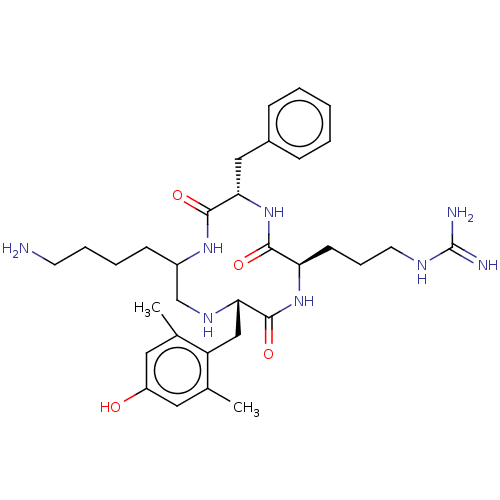 (CHEMBL3904924) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description . Binding affinity for Opioid receptor mu 1 determined by displacing [3H]-DAMGO from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198756 (CHEMBL3951723) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198755 (CHEMBL3979449) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198757 (CHEMBL363142) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198756 (CHEMBL3951723) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198759 (CHEMBL3904924) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080455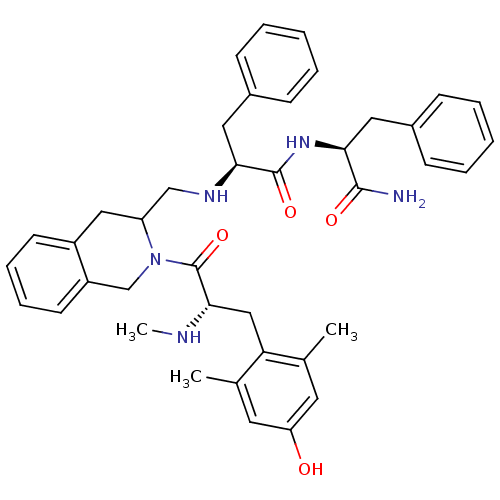 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-({2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080455 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-({2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description . Binding affinity for Opioid receptor mu 1 determined by displacing [3H]-DAMGO from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080452 ((S)-2-({2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description . Binding affinity for Opioid receptor mu 1 determined by displacing [3H]-DAMGO from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50252861 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198752 (CHEMBL3976694) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198752 (CHEMBL3976694) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198759 (CHEMBL3904924) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 422 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198753 (CHEMBL3944636) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50080452 ((S)-2-({2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propio...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor kappa 1 determined by displacement of [3H]U69,593 from guinea pig brain membranes | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50080454 (2-[(S)-3-(4-Hydroxy-2,6-dimethyl-phenyl)-2-methyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor kappa 1 determined by displacement of [3H]U69,593 from guinea pig brain membranes | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50080455 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-({2-[(S)-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor kappa 1 determined by displacement of [3H]U69,593 from guinea pig brain membranes | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50080453 ((S)-2-({2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-p...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor kappa 1 determined by displacement of [3H]U69,593 from guinea pig brain membranes | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001157 (CHEMBL538700) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor kappa 1 determined by displacement of [3H]U69,593 from guinea pig brain membranes | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198753 (CHEMBL3944636) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198757 (CHEMBL363142) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50252861 (CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50198757 (CHEMBL363142) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Agonist activity at MOR in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contractions | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50198757 (CHEMBL363142) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Antagonist activity at MOR in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |