

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50005520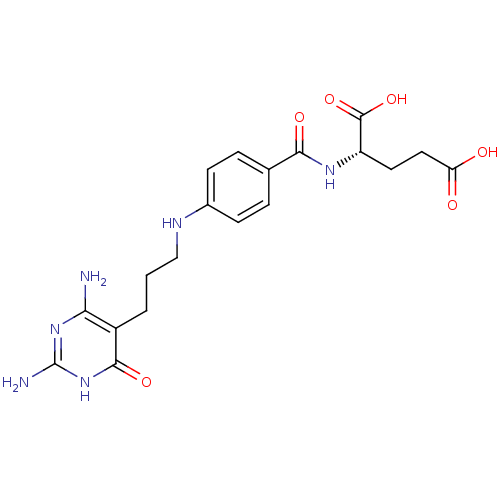 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Hexaglutamyl homologue inhibition activity against the AICAR formyltransferase was determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against Glycinamide ribonucleotide transformylase(GAR-TFase) against hog liver | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Triglutamyl homologue inhibition activity against AICAR formyltransferase was determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against MOLT-4 | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50014844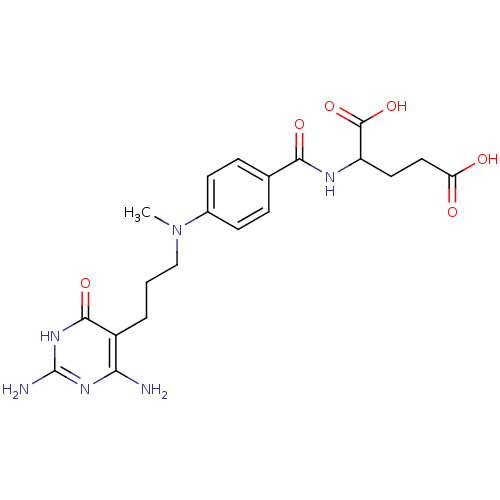 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against Glycinamide ribonucleotide transformylase(GAR-TFase) from L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50014843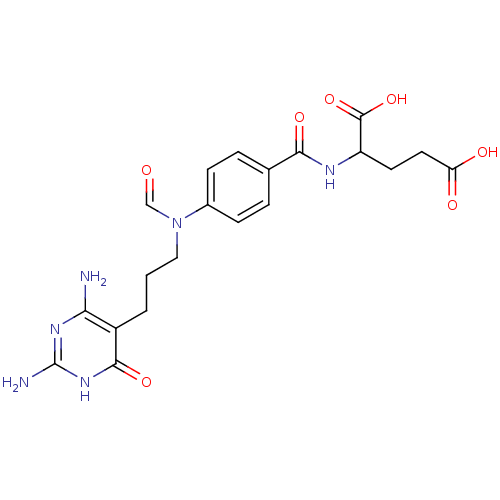 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50014843 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against Glycinamide ribonucleotide transformylase(GAR-TFase) against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50014844 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase from hog liver | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against MOLT-4 | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against MOLT-4 | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50014843 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50014843 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Hexaglutamyl homologue inhibition activity against the AICAR formyltransferase was determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||