

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50030815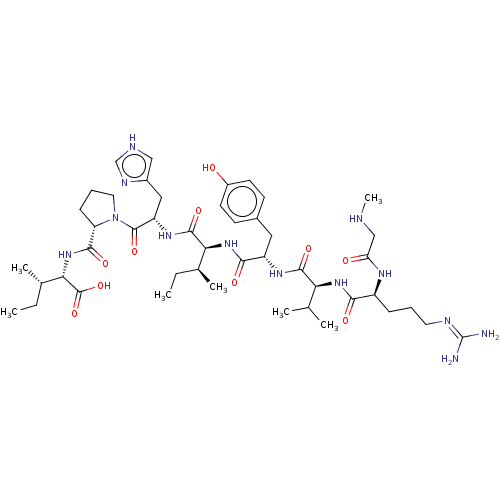 (CHEMBL404594) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company Curated by ChEMBL | Assay Description Binding affinity for rat brain Angiotensin II receptor | J Med Chem 34: 3248-60 (1991) BindingDB Entry DOI: 10.7270/Q24X5B06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006848 (CHEMBL88091 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006850 (CHEMBL313063 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006858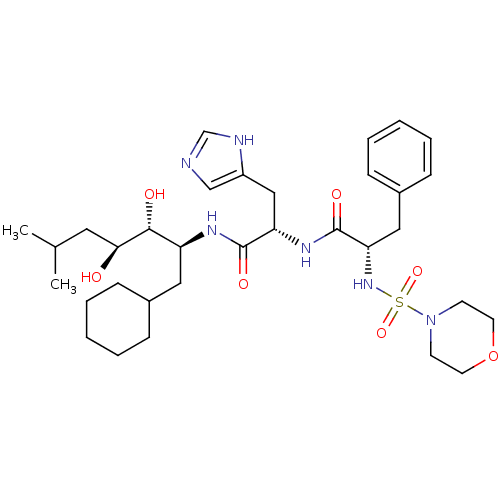 (CHEMBL315380 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006854 (CHEMBL88069 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006853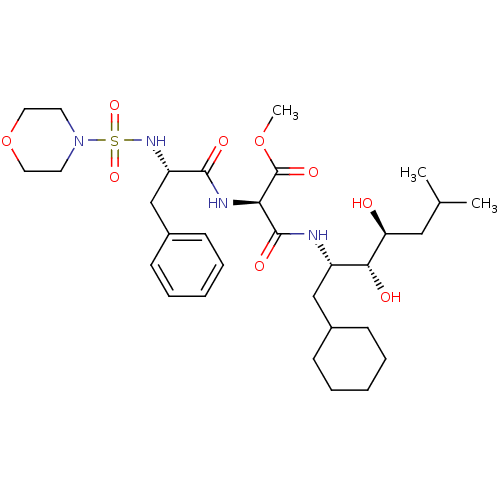 (CHEMBL315211 | N-(1-Cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50030815 (CHEMBL404594) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Angiotensin II receptor, from rat adrenal gland | J Med Chem 34: 3248-60 (1991) BindingDB Entry DOI: 10.7270/Q24X5B06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006845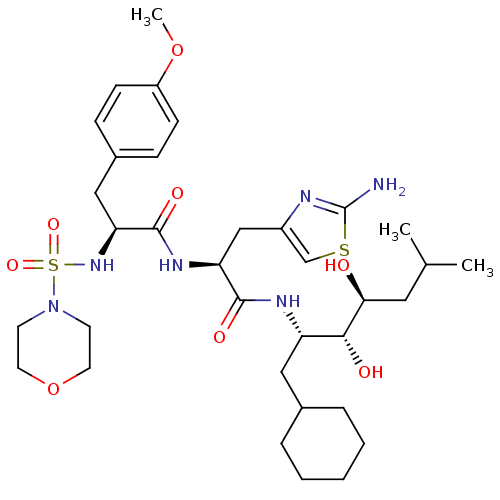 (CHEMBL316208 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844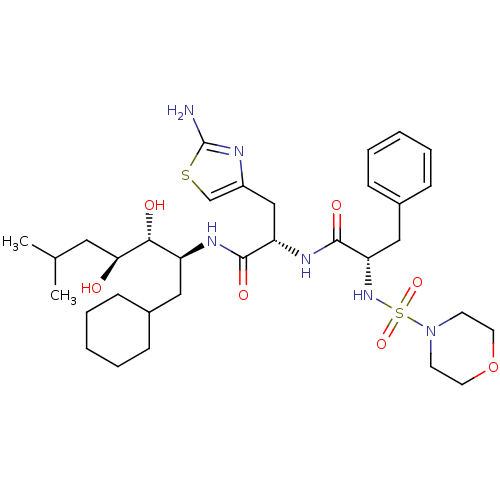 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of plasma renin activity in monkey | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006851 (CHEMBL263531 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009873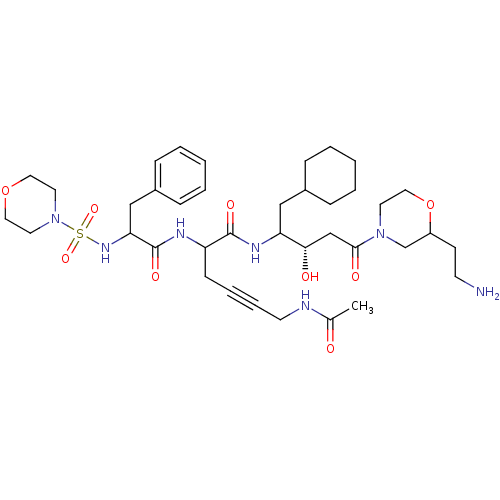 (6-Acetylamino-2-[2-(morpholine-4-sulfonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006856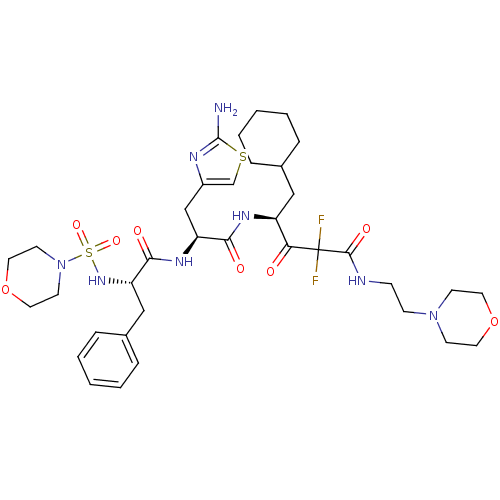 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006846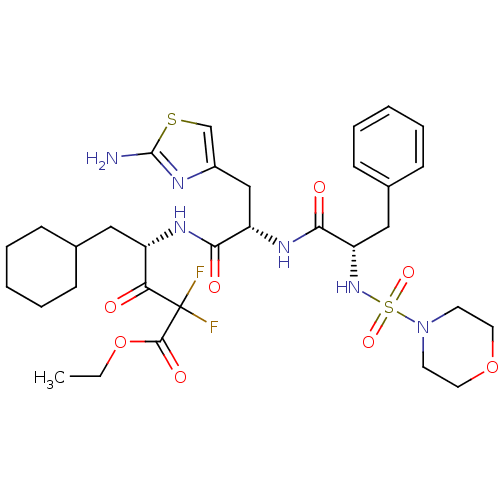 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of plasma renin activity in human | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006843 (CHEMBL314066 | N-[(2-Amino-thiazol-4-yl)-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.581 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006849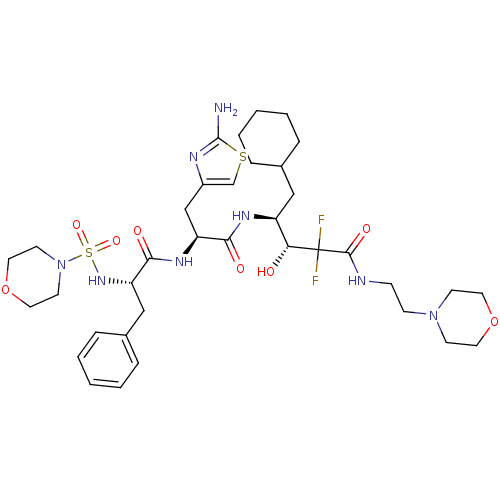 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006202 (3-Amino-N-[1-[1-(1-cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17941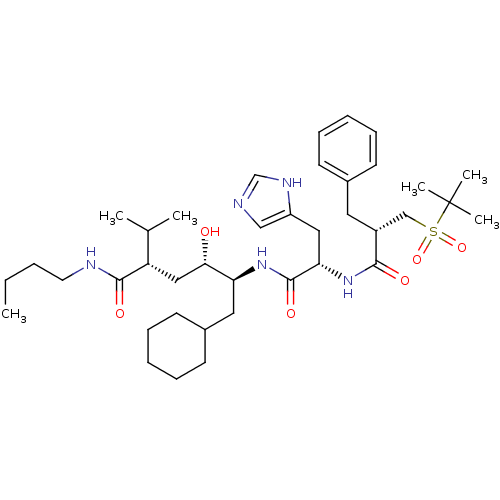 ((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006860 (CHEMBL85672 | {1-[2-(2-Amino-thiazol-4-yl)-1-(1-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50228195 (Angiotensin Ii | CHEBI:2719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Angiotensin II receptor, from rat adrenal gland | J Med Chem 34: 3248-60 (1991) BindingDB Entry DOI: 10.7270/Q24X5B06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50228894 (CHEMBL312754) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Angiotensin II receptor, from rat adrenal gland | J Med Chem 34: 3248-60 (1991) BindingDB Entry DOI: 10.7270/Q24X5B06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009879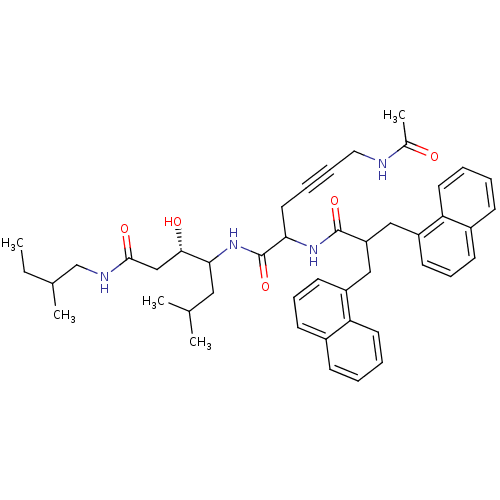 (4-[6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of plasma renin activity in monkey | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006864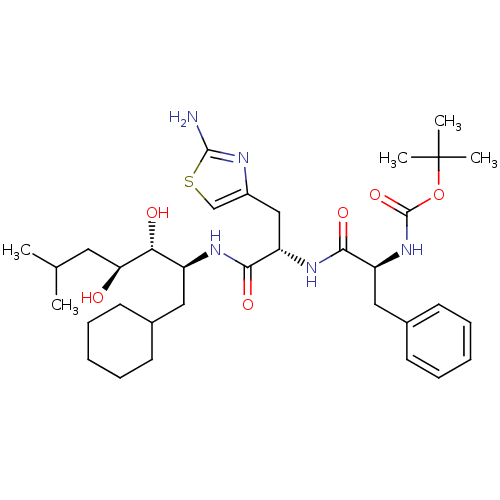 (CHEMBL420417 | {1-[2-(2-Amino-thiazol-4-yl)-1-(1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009911 (6-(3-Methyl-thioureido)-2-[2-(morpholine-4-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006859 (2-((R)-(S)-Morpholine-4-sulfonyl)-1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009918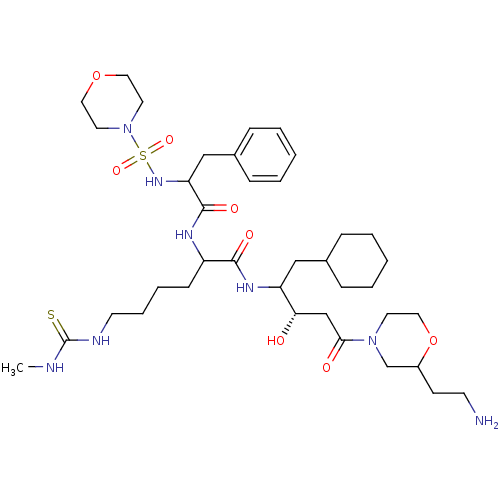 (6-(3-Methyl-thioureido)-2-[2-(morpholine-4-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009900 (CHEMBL23944 | {5-{4-[2-(2-Amino-ethyl)-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009885 (6-(3-Methyl-thioureido)-2-[2-(morpholine-4-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006861 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006857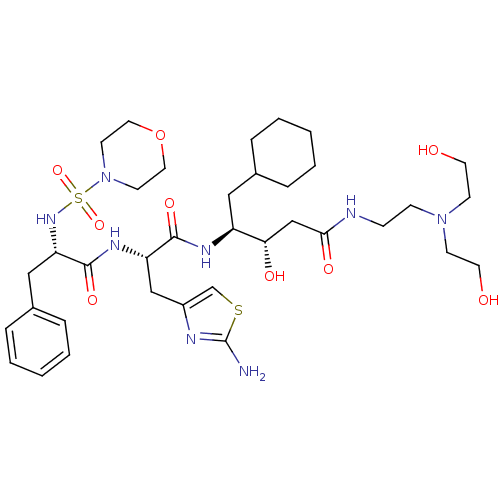 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50228195 (Angiotensin Ii | CHEBI:2719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company Curated by ChEMBL | Assay Description Binding affinity for rat brain Angiotensin II receptor | J Med Chem 34: 3248-60 (1991) BindingDB Entry DOI: 10.7270/Q24X5B06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006852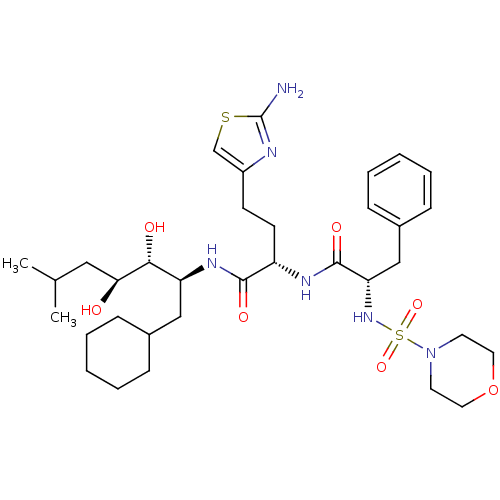 (4-(2-Amino-thiazol-4-yl)-N-(1-cyclohexylmethyl-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009879 (4-[6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009879 (4-[6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009884 (CHEMBL279921 | N-[1-{4-[2-(2-Amino-ethyl)-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50228894 (CHEMBL312754) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company Curated by ChEMBL | Assay Description Binding affinity for rat brain Angiotensin II receptor | J Med Chem 34: 3248-60 (1991) BindingDB Entry DOI: 10.7270/Q24X5B06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009884 (CHEMBL279921 | N-[1-{4-[2-(2-Amino-ethyl)-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50224920 (3-methoxy-5-(2-methyl-1,6-naphthyridin-7-yl)benzon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Antagonist activity at rat mGlu5 receptor expressed in CHO cells assessed as inhibition of quisqualate-stimulated calcium mobilization | Bioorg Med Chem Lett 17: 6525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.083 BindingDB Entry DOI: 10.7270/Q2V40TXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009872 (4-[6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3451 (3-tert-butyl-1-[6-(2,6-dichlorophenyl)-2-{[4-(diet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3453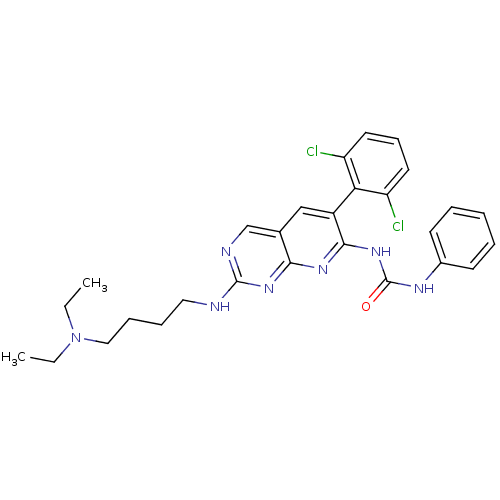 (3-[6-(2,6-dichlorophenyl)-2-{[4-(diethylamino)buty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009915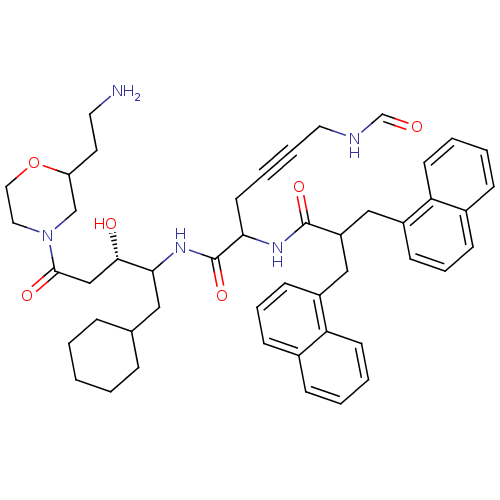 (6-Formylamino-2-(3-naphthalen-1-yl-2-naphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009916 (6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3467 (3-tert-butyl-1-[6-(2,6-dichlorophenyl)-2-{[4-(4-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009892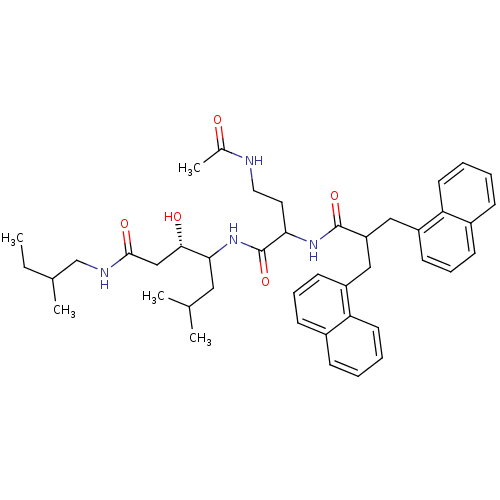 (4-[4-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009894 (6-(N'-nitro-guanidino)-2-(3-naphthalen-1-yl-2-naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3452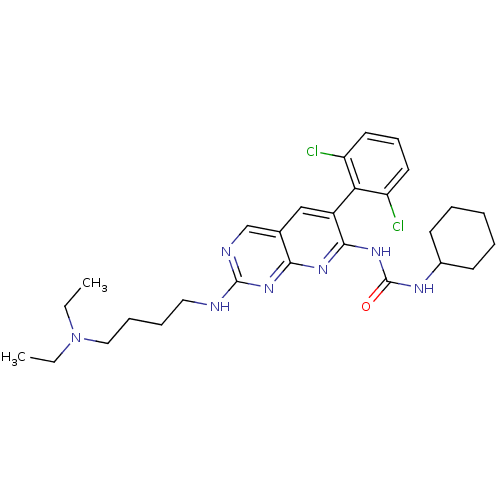 (1-cyclohexyl-3-[6-(2,6-dichlorophenyl)-2-{[4-(diet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009905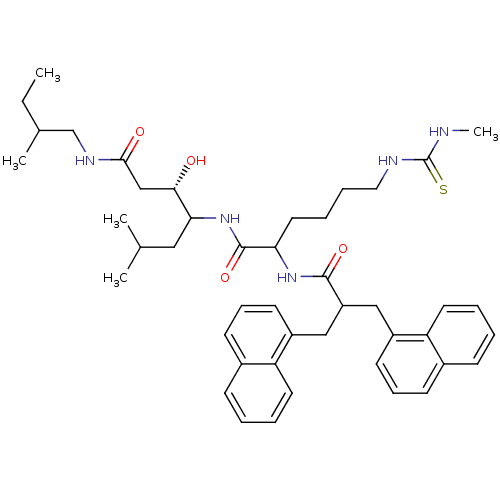 (3-Hydroxy-6-methyl-4-[6-(3-methyl-thioureido)-2-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 710 total ) | Next | Last >> |