 Found 103 hits with Last Name = 'cook' and Initial = 'tr'
Found 103 hits with Last Name = 'cook' and Initial = 'tr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124519

(5,8-Difluoro-2-furan-2-yl-1,2-dihydro-quinazolin-4...)Show InChI InChI=1S/C12H9F2N3O/c13-6-3-4-7(14)10-9(6)11(15)17-12(16-10)8-2-1-5-18-8/h1-5,12,16H,(H2,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339998
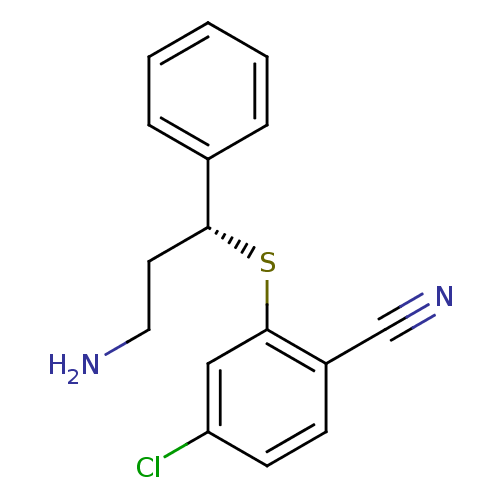
((R)-2-(3-amino-1-phenylpropylthio)-4-chlorobenzoni...)Show InChI InChI=1S/C16H15ClN2S/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339997
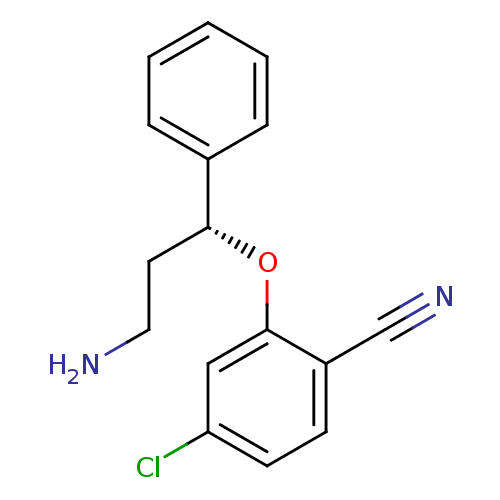
((R)-2-(3-amino-1-phenylpropoxy)-4-chlorobenzonitri...)Show InChI InChI=1S/C16H15ClN2O/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340003

((R)-2-(3-amino-1-phenylpropylthio)-6-methylnicotin...)Show InChI InChI=1S/C16H17N3S/c1-12-7-8-14(11-18)16(19-12)20-15(9-10-17)13-5-3-2-4-6-13/h2-8,15H,9-10,17H2,1H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340004
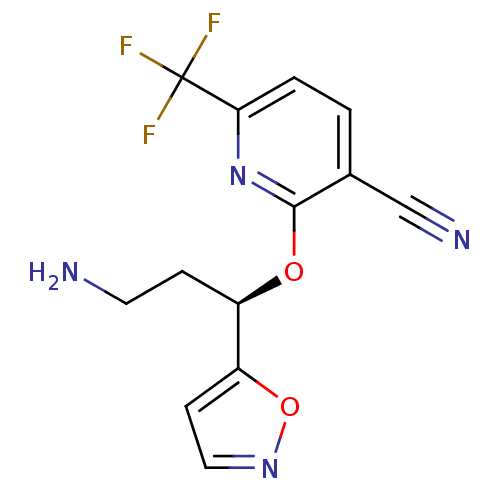
((R)-2-(3-amino-1-(isoxazol-5-yl)propoxy)-6-(triflu...)Show SMILES NCC[C@@H](Oc1nc(ccc1C#N)C(F)(F)F)c1ccno1 |r| Show InChI InChI=1S/C13H11F3N4O2/c14-13(15,16)11-2-1-8(7-18)12(20-11)21-9(3-5-17)10-4-6-19-22-10/h1-2,4,6,9H,3,5,17H2/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340002

(2-[[(1R)-3-Amino-1-phenylpropyl]oxy]-4-chloro-5-fl...)Show InChI InChI=1S/C16H14ClFN2O/c17-13-9-16(12(10-20)8-14(13)18)21-15(6-7-19)11-4-2-1-3-5-11/h1-5,8-9,15H,6-7,19H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339996

((R)-4-chloro-2-(3-(methylamino)-1-phenylpropoxy)be...)Show InChI InChI=1S/C17H17ClN2O/c1-20-10-9-16(13-5-3-2-4-6-13)21-17-11-15(18)8-7-14(17)12-19/h2-8,11,16,20H,9-10H2,1H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124537
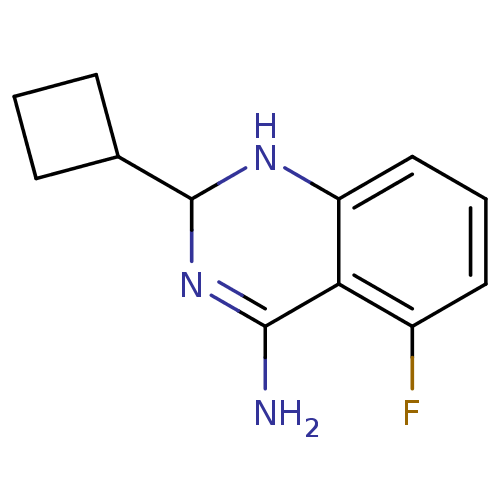
(2-Cyclobutyl-5-fluoro-1,2-dihydro-quinazolin-4-yla...)Show InChI InChI=1S/C12H14FN3/c13-8-5-2-6-9-10(8)11(14)16-12(15-9)7-3-1-4-7/h2,5-7,12,15H,1,3-4H2,(H2,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339995
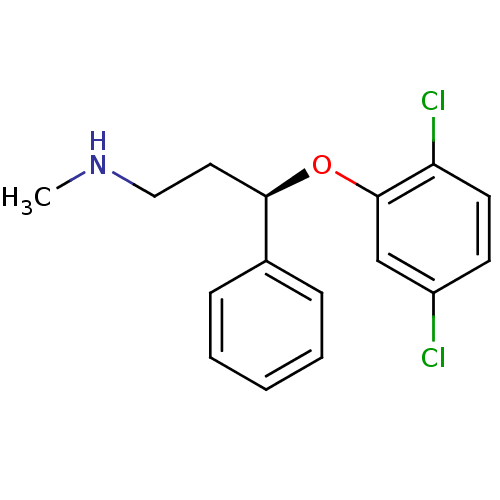
((R)-3-(2,5-dichlorophenoxy)-N-methyl-3-phenylpropa...)Show InChI InChI=1S/C16H17Cl2NO/c1-19-10-9-15(12-5-3-2-4-6-12)20-16-11-13(17)7-8-14(16)18/h2-8,11,15,19H,9-10H2,1H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124531

(4-[4'-amino-5'-fluorospiro[hexahydropyridine-4,2'-...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(cc2)C#N)Nc2cccc(F)c12 |t:1| Show InChI InChI=1S/C20H18FN5O/c21-15-2-1-3-16-17(15)18(23)25-20(24-16)8-10-26(11-9-20)19(27)14-6-4-13(12-22)5-7-14/h1-7,24H,8-11H2,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124521

(CHEMBL174290 | ethyl 4'-amino-5'-fluorospiro[hexah...)Show SMILES CCOC(=O)N1CCC2(CC1)Nc1cccc(F)c1C(N)=N2 |c:22| Show InChI InChI=1S/C15H19FN4O2/c1-2-22-14(21)20-8-6-15(7-9-20)18-11-5-3-4-10(16)12(11)13(17)19-15/h3-5,18H,2,6-9H2,1H3,(H2,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124535
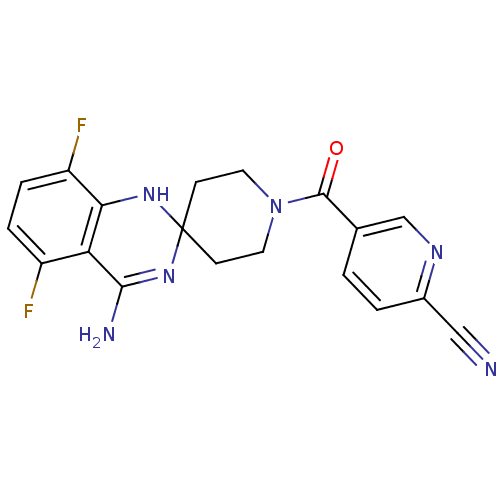
(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124533

(2-methylsulfanylethyl 4'-amino-5'-fluorospiro[hexa...)Show SMILES CSCCOC(=O)N1CCC2(CC1)Nc1cccc(F)c1C(N)=N2 |c:24| Show InChI InChI=1S/C16H21FN4O2S/c1-24-10-9-23-15(22)21-7-5-16(6-8-21)19-12-4-2-3-11(17)13(12)14(18)20-16/h2-4,19H,5-10H2,1H3,(H2,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124535

(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124532
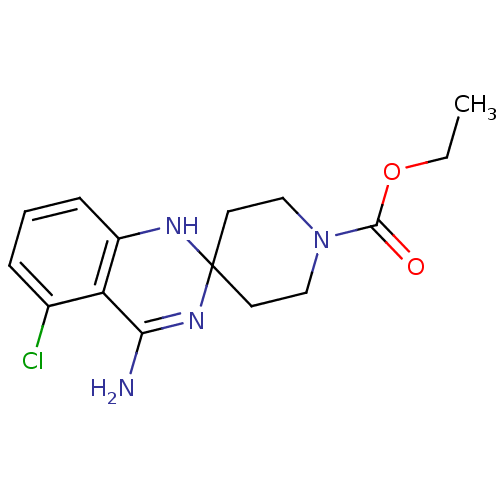
(CHEMBL366978 | ethyl 4'-amino-5'-chlorospiro[hexah...)Show SMILES CCOC(=O)N1CCC2(CC1)Nc1cccc(Cl)c1C(N)=N2 |c:22| Show InChI InChI=1S/C15H19ClN4O2/c1-2-22-14(21)20-8-6-15(7-9-20)18-11-5-3-4-10(16)12(11)13(17)19-15/h3-5,18H,2,6-9H2,1H3,(H2,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124520
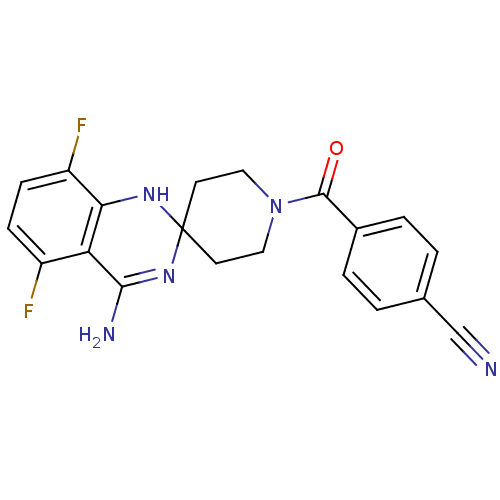
(4-[4'-amino-5',8'-difluorospiro[hexahydropyridine-...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(cc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C20H17F2N5O/c21-14-5-6-15(22)17-16(14)18(24)26-20(25-17)7-9-27(10-8-20)19(28)13-3-1-12(11-23)2-4-13/h1-6,25H,7-10H2,(H2,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124529

(5-Fluoro-2-(4-fluoro-phenyl)-1,2-dihydro-quinazoli...)Show InChI InChI=1S/C14H11F2N3/c15-9-6-4-8(5-7-9)14-18-11-3-1-2-10(16)12(11)13(17)19-14/h1-7,14,18H,(H2,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340001
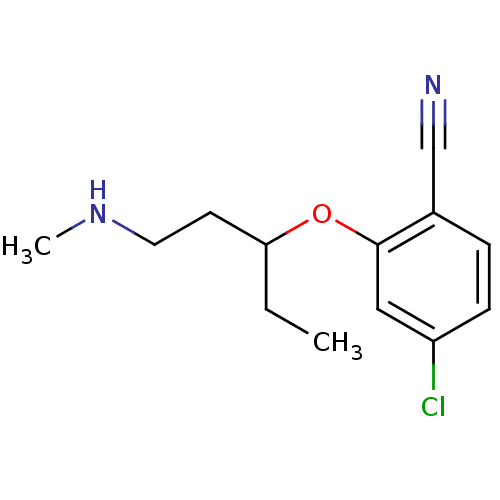
(CHEMBL1762478 | rac-4-chloro-2-(1-(methylamino)pen...)Show InChI InChI=1S/C13H17ClN2O/c1-3-12(6-7-16-2)17-13-8-11(14)5-4-10(13)9-15/h4-5,8,12,16H,3,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50124519

(5,8-Difluoro-2-furan-2-yl-1,2-dihydro-quinazolin-4...)Show InChI InChI=1S/C12H9F2N3O/c13-6-3-4-7(14)10-9(6)11(15)17-12(16-10)8-2-1-5-18-8/h1-5,12,16H,(H2,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124525
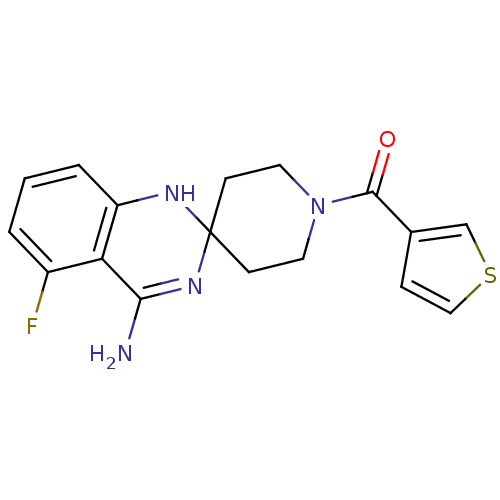
(4'-amino-5'-fluorospiro[hexahydropyridine-4,2'-(1'...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccsc2)Nc2cccc(F)c12 |t:1| Show InChI InChI=1S/C17H17FN4OS/c18-12-2-1-3-13-14(12)15(19)21-17(20-13)5-7-22(8-6-17)16(23)11-4-9-24-10-11/h1-4,9-10,20H,5-8H2,(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339999
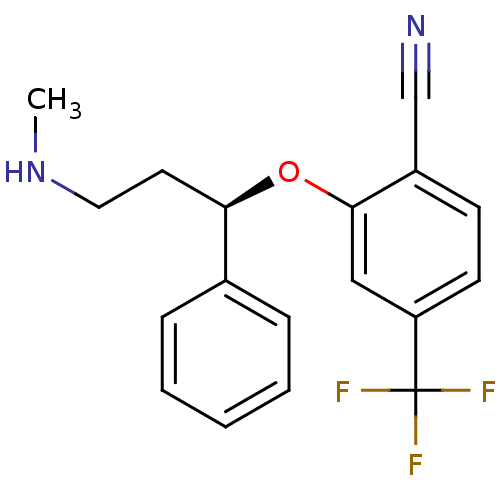
((R)-2-(3-(methylamino)-1-phenylpropoxy)-4-(trifluo...)Show SMILES CNCC[C@@H](Oc1cc(ccc1C#N)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C18H17F3N2O/c1-23-10-9-16(13-5-3-2-4-6-13)24-17-11-15(18(19,20)21)8-7-14(17)12-22/h2-8,11,16,23H,9-10H2,1H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50030279
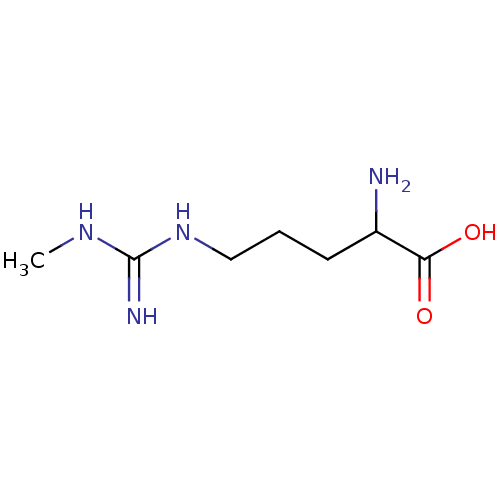
(2-Amino-5-(N'-methyl-guanidino)-pentanoic acid | C...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50124535

(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50063300
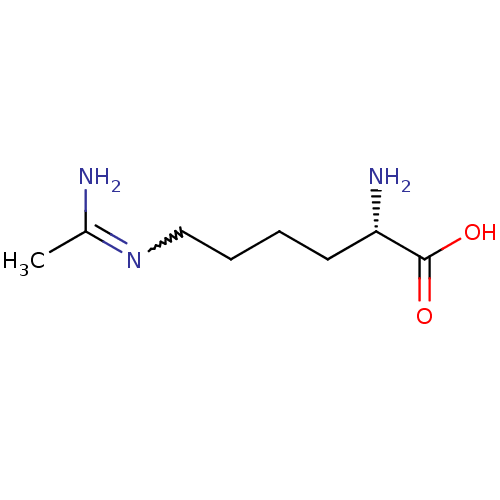
((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340000
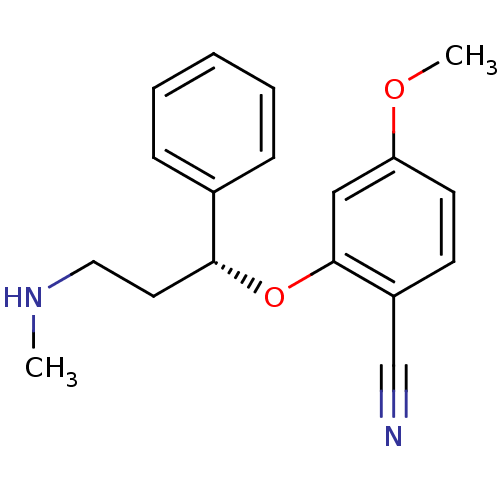
((R)-4-methoxy-2-(3-(methylamino)-1-phenylpropoxy)b...)Show InChI InChI=1S/C18H20N2O2/c1-20-11-10-17(14-6-4-3-5-7-14)22-18-12-16(21-2)9-8-15(18)13-19/h3-9,12,17,20H,10-11H2,1-2H3/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50098937
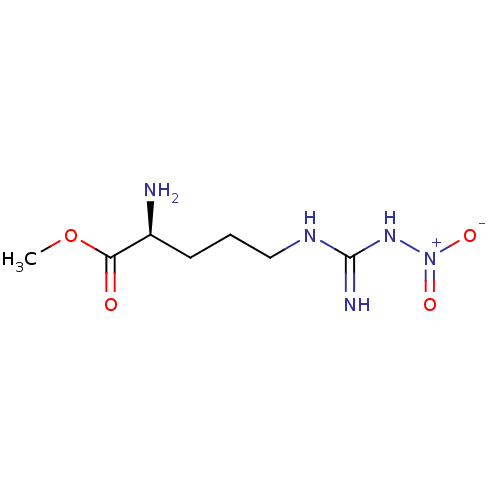
((S)-methyl 2-amino-5-(amino(nitroamino)methyleneam...)Show SMILES COC(=O)[C@@H](N)CCCNC(N)=N[N+]([O-])=O |r,w:12.12| Show InChI InChI=1S/C7H15N5O4/c1-16-6(13)5(8)3-2-4-10-7(9)11-12(14)15/h5H,2-4,8H2,1H3,(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50124519

(5,8-Difluoro-2-furan-2-yl-1,2-dihydro-quinazolin-4...)Show InChI InChI=1S/C12H9F2N3O/c13-6-3-4-7(14)10-9(6)11(15)17-12(16-10)8-2-1-5-18-8/h1-5,12,16H,(H2,15,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339998

((R)-2-(3-amino-1-phenylpropylthio)-4-chlorobenzoni...)Show InChI InChI=1S/C16H15ClN2S/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in intact human DLD1 cells assessed as nitric oxide production |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124526
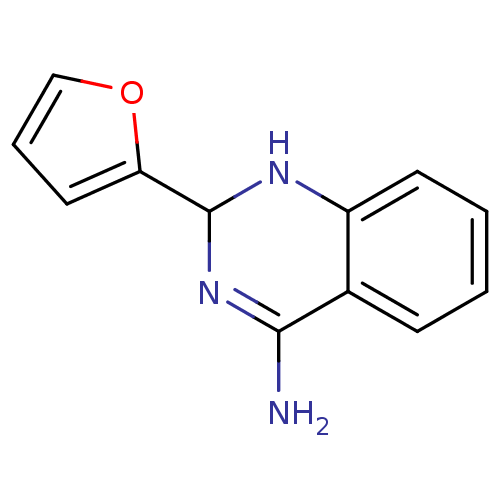
(2-Furan-2-yl-1,2-dihydro-quinazolin-4-ylamine | CH...)Show InChI InChI=1S/C12H11N3O/c13-11-8-4-1-2-5-9(8)14-12(15-11)10-6-3-7-16-10/h1-7,12,14H,(H2,13,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124528
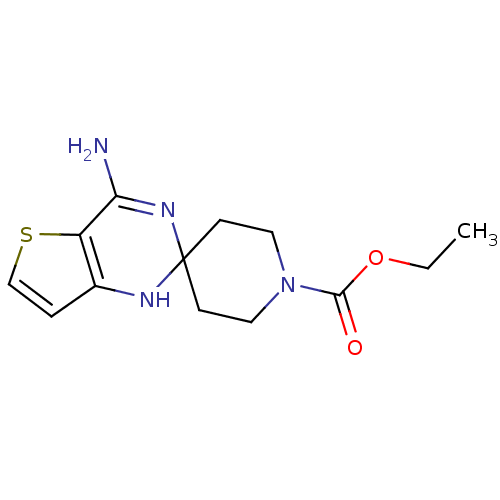
(CHEMBL170176 | ethyl 4'-amino-1H,1'H-spiro[piperid...)Show InChI InChI=1S/C13H18N4O2S/c1-2-19-12(18)17-6-4-13(5-7-17)15-9-3-8-20-10(9)11(14)16-13/h3,8,15H,2,4-7H2,1H3,(H2,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50340000

((R)-4-methoxy-2-(3-(methylamino)-1-phenylpropoxy)b...)Show InChI InChI=1S/C18H20N2O2/c1-20-11-10-17(14-6-4-3-5-7-14)22-18-12-16(21-2)9-8-15(18)13-19/h3-9,12,17,20H,10-11H2,1-2H3/t17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50030279

(2-Amino-5-(N'-methyl-guanidino)-pentanoic acid | C...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50030279

(2-Amino-5-(N'-methyl-guanidino)-pentanoic acid | C...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50340002

(2-[[(1R)-3-Amino-1-phenylpropyl]oxy]-4-chloro-5-fl...)Show InChI InChI=1S/C16H14ClFN2O/c17-13-9-16(12(10-20)8-14(13)18)21-15(6-7-19)11-4-2-1-3-5-11/h1-5,8-9,15H,6-7,19H2/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124527
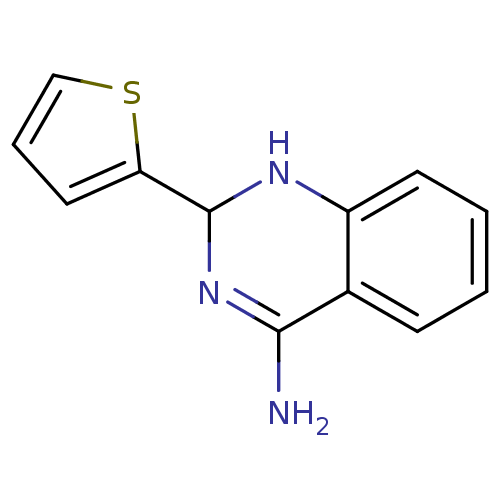
(2-Thiophen-2-yl-1,2-dihydro-quinazolin-4-ylamine |...)Show InChI InChI=1S/C12H11N3S/c13-11-8-4-1-2-5-9(8)14-12(15-11)10-6-3-7-16-10/h1-7,12,14H,(H2,13,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50340001

(CHEMBL1762478 | rac-4-chloro-2-(1-(methylamino)pen...)Show InChI InChI=1S/C13H17ClN2O/c1-3-12(6-7-16-2)17-13-8-11(14)5-4-10(13)9-15/h4-5,8,12,16H,3,6-7H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50339997

((R)-2-(3-amino-1-phenylpropoxy)-4-chlorobenzonitri...)Show InChI InChI=1S/C16H15ClN2O/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50339996

((R)-4-chloro-2-(3-(methylamino)-1-phenylpropoxy)be...)Show InChI InChI=1S/C17H17ClN2O/c1-20-10-9-16(13-5-3-2-4-6-13)21-17-11-15(18)8-7-14(17)12-19/h2-8,11,16,20H,9-10H2,1H3/t16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340003

((R)-2-(3-amino-1-phenylpropylthio)-6-methylnicotin...)Show InChI InChI=1S/C16H17N3S/c1-12-7-8-14(11-18)16(19-12)20-15(9-10-17)13-5-3-2-4-6-13/h2-8,15H,9-10,17H2,1H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in intact human DLD1 cells assessed as nitric oxide production |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340002

(2-[[(1R)-3-Amino-1-phenylpropyl]oxy]-4-chloro-5-fl...)Show InChI InChI=1S/C16H14ClFN2O/c17-13-9-16(12(10-20)8-14(13)18)21-15(6-7-19)11-4-2-1-3-5-11/h1-5,8-9,15H,6-7,19H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in intact human DLD1 cells assessed as nitric oxide production |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339996

((R)-4-chloro-2-(3-(methylamino)-1-phenylpropoxy)be...)Show InChI InChI=1S/C17H17ClN2O/c1-20-10-9-16(13-5-3-2-4-6-13)21-17-11-15(18)8-7-14(17)12-19/h2-8,11,16,20H,9-10H2,1H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in intact human DLD1 cells assessed as nitric oxide production |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340004

((R)-2-(3-amino-1-(isoxazol-5-yl)propoxy)-6-(triflu...)Show SMILES NCC[C@@H](Oc1nc(ccc1C#N)C(F)(F)F)c1ccno1 |r| Show InChI InChI=1S/C13H11F3N4O2/c14-13(15,16)11-2-1-8(7-18)12(20-11)21-9(3-5-17)10-4-6-19-22-10/h1-2,4,6,9H,3,5,17H2/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in intact human DLD1 cells assessed as nitric oxide production |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339994
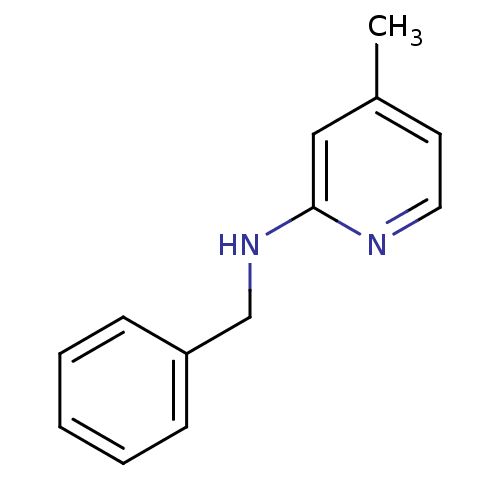
(CHEMBL1762482 | N-benzyl-4-methylpyridin-2-amine)Show InChI InChI=1S/C13H14N2/c1-11-7-8-14-13(9-11)15-10-12-5-3-2-4-6-12/h2-9H,10H2,1H3,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339997

((R)-2-(3-amino-1-phenylpropoxy)-4-chlorobenzonitri...)Show InChI InChI=1S/C16H15ClN2O/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in intact human DLD1 cells assessed as nitric oxide production |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124530
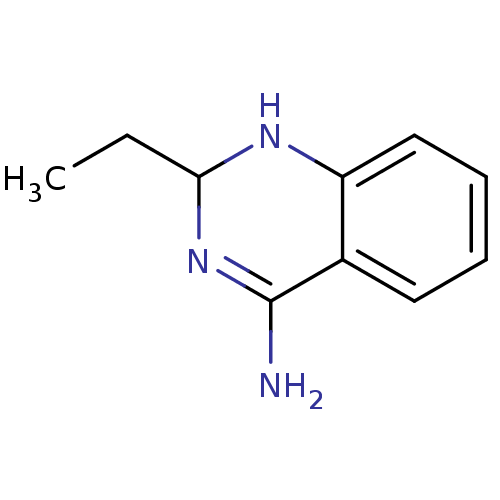
(2-Ethyl-1,2-dihydro-quinazolin-4-ylamine | CHEMBL1...)Show InChI InChI=1S/C10H13N3/c1-2-9-12-8-6-4-3-5-7(8)10(11)13-9/h3-6,9,12H,2H2,1H3,(H2,11,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340006

((R)-2-(3-amino-1-phenylpropoxy)-5-chlorobenzonitri...)Show InChI InChI=1S/C16H15ClN2O/c17-14-6-7-15(13(10-14)11-19)20-16(8-9-18)12-4-2-1-3-5-12/h1-7,10,16H,8-9,18H2/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124535

(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in intact human DLD1 cells assessed as nitric oxide production |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50124535

(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50339996

((R)-4-chloro-2-(3-(methylamino)-1-phenylpropoxy)be...)Show InChI InChI=1S/C17H17ClN2O/c1-20-10-9-16(13-5-3-2-4-6-13)21-17-11-15(18)8-7-14(17)12-19/h2-8,11,16,20H,9-10H2,1H3/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50124537

(2-Cyclobutyl-5-fluoro-1,2-dihydro-quinazolin-4-yla...)Show InChI InChI=1S/C12H14FN3/c13-8-5-2-6-9-10(8)11(14)16-12(15-9)7-3-1-4-7/h2,5-7,12,15H,1,3-4H2,(H2,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data