 Found 365 hits with Last Name = 'cooper' and Initial = 'aw'
Found 365 hits with Last Name = 'cooper' and Initial = 'aw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50521218
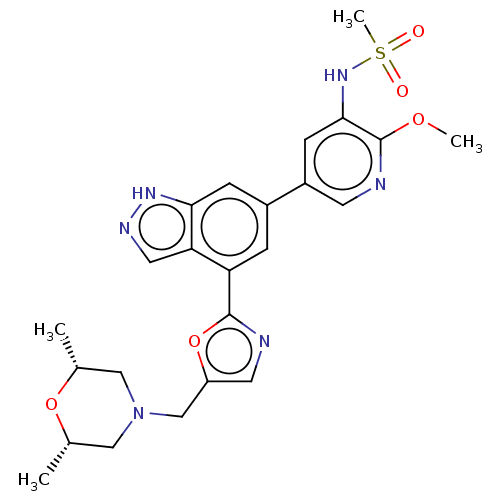
(CHEMBL4434674)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1cc(-c2ncc(CN3C[C@H](C)O[C@H](C)C3)o2)c2cn[nH]c2c1 |r| Show InChI InChI=1S/C24H28N6O5S/c1-14-11-30(12-15(2)34-14)13-18-9-26-23(35-18)19-5-16(6-21-20(19)10-27-28-21)17-7-22(29-36(4,31)32)24(33-3)25-8-17/h5-10,14-15,29H,11-13H2,1-4H3,(H,27,28)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins by HT... |
J Med Chem 61: 11061-11073 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01556
BindingDB Entry DOI: 10.7270/Q2Z60SFQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50004529
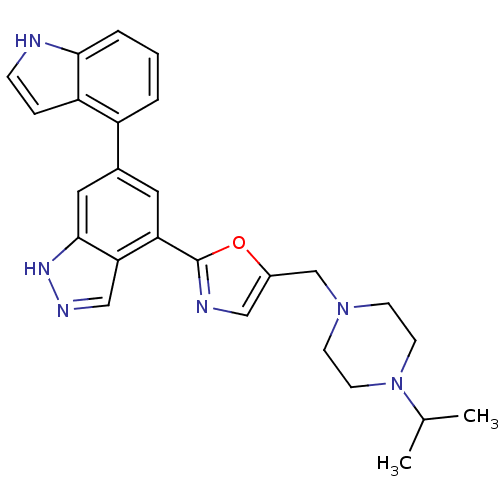
(CHEMBL2216859)Show SMILES CC(C)N1CCN(Cc2cnc(o2)-c2cc(cc3[nH]ncc23)-c2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C26H28N6O/c1-17(2)32-10-8-31(9-11-32)16-19-14-28-26(33-19)22-12-18(13-25-23(22)15-29-30-25)20-4-3-5-24-21(20)6-7-27-24/h3-7,12-15,17,27H,8-11,16H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins by HT... |
J Med Chem 61: 11061-11073 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01556
BindingDB Entry DOI: 10.7270/Q2Z60SFQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470598
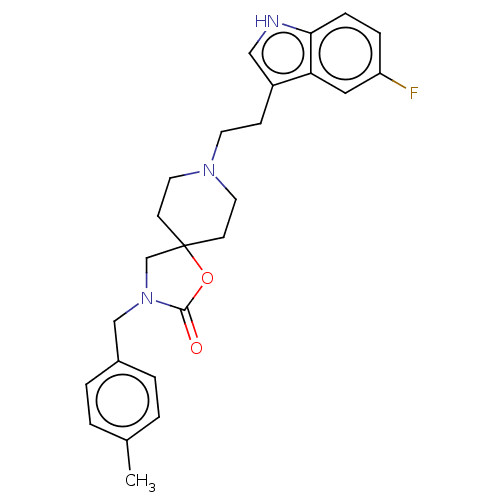
(CHEMBL126050)Show SMILES Cc1ccc(CN2CC3(CCN(CCc4c[nH]c5ccc(F)cc45)CC3)OC2=O)cc1 Show InChI InChI=1S/C25H28FN3O2/c1-18-2-4-19(5-3-18)16-29-17-25(31-24(29)30)9-12-28(13-10-25)11-8-20-15-27-23-7-6-21(26)14-22(20)23/h2-7,14-15,27H,8-13,16-17H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470591
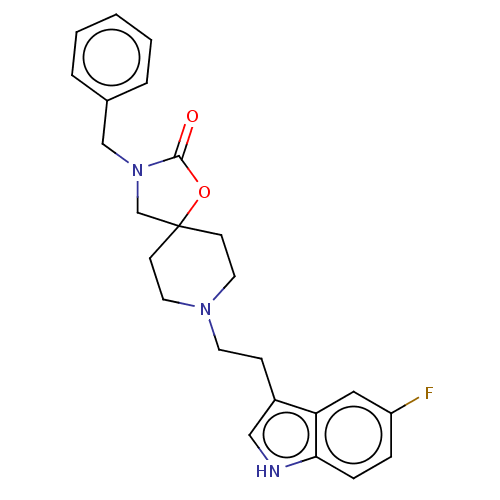
(CHEMBL124208)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccccc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C24H26FN3O2/c25-20-6-7-22-21(14-20)19(15-26-22)8-11-27-12-9-24(10-13-27)17-28(23(29)30-24)16-18-4-2-1-3-5-18/h1-7,14-15,26H,8-13,16-17H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470590
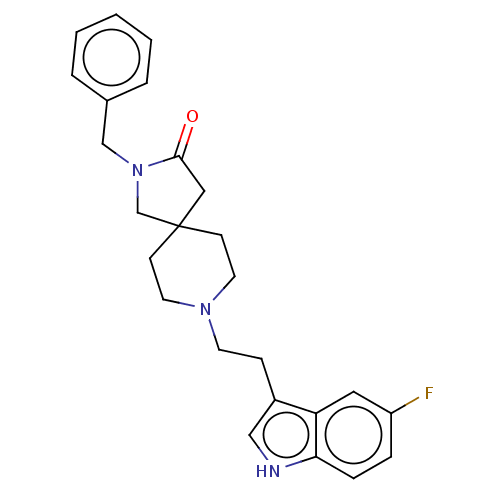
(CHEMBL341357)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccccc5)C(=O)C4)CC3)c2c1 Show InChI InChI=1S/C25H28FN3O/c26-21-6-7-23-22(14-21)20(16-27-23)8-11-28-12-9-25(10-13-28)15-24(30)29(18-25)17-19-4-2-1-3-5-19/h1-7,14,16,27H,8-13,15,17-18H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470604
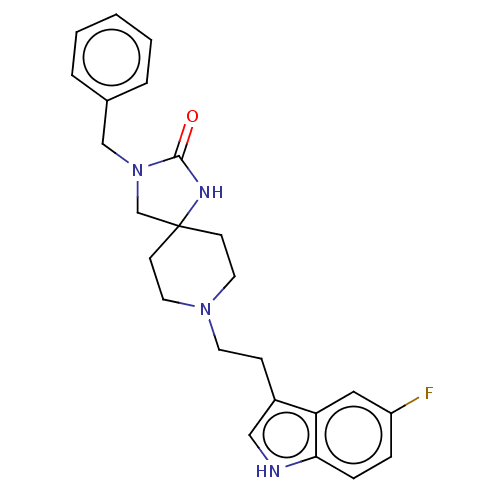
(CHEMBL338825)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccccc5)C(=O)N4)CC3)c2c1 Show InChI InChI=1S/C24H27FN4O/c25-20-6-7-22-21(14-20)19(15-26-22)8-11-28-12-9-24(10-13-28)17-29(23(30)27-24)16-18-4-2-1-3-5-18/h1-7,14-15,26H,8-13,16-17H2,(H,27,30) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470596

(CHEMBL125310)Show SMILES CC(=O)N(Cc1ccccc1)CC1(O)CCN(CCc2c[nH]c3ccc(F)cc23)CC1 Show InChI InChI=1S/C25H30FN3O2/c1-19(30)29(17-20-5-3-2-4-6-20)18-25(31)10-13-28(14-11-25)12-9-21-16-27-24-8-7-22(26)15-23(21)24/h2-8,15-16,27,31H,9-14,17-18H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470583
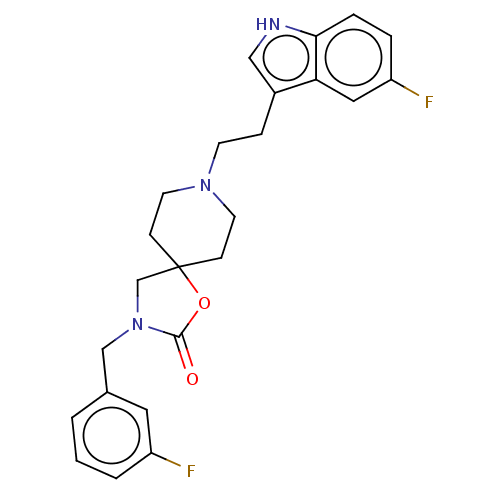
(CHEMBL125696)Show SMILES Fc1cccc(CN2CC3(CCN(CCc4c[nH]c5ccc(F)cc45)CC3)OC2=O)c1 Show InChI InChI=1S/C24H25F2N3O2/c25-19-3-1-2-17(12-19)15-29-16-24(31-23(29)30)7-10-28(11-8-24)9-6-18-14-27-22-5-4-20(26)13-21(18)22/h1-5,12-14,27H,6-11,15-16H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470587
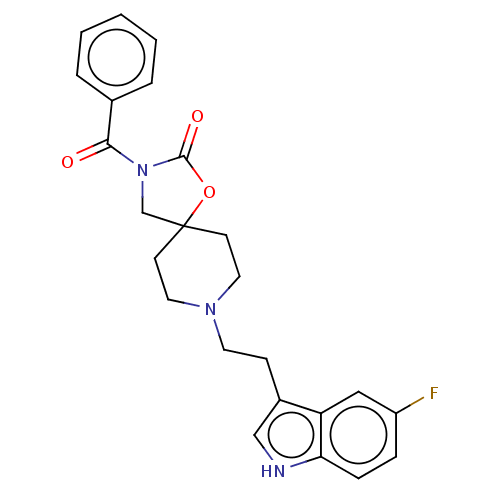
(CHEMBL124648)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(C(=O)c5ccccc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C24H24FN3O3/c25-19-6-7-21-20(14-19)18(15-26-21)8-11-27-12-9-24(10-13-27)16-28(23(30)31-24)22(29)17-4-2-1-3-5-17/h1-7,14-15,26H,8-13,16H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM50470591

(CHEMBL124208)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccccc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C24H26FN3O2/c25-20-6-7-22-21(14-20)19(15-26-22)8-11-27-12-9-24(10-13-27)17-28(23(29)30-24)16-18-4-2-1-3-5-18/h1-7,14-15,26H,8-13,16-17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against Tachykinin receptor 2 from guinea pig trachea |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470605
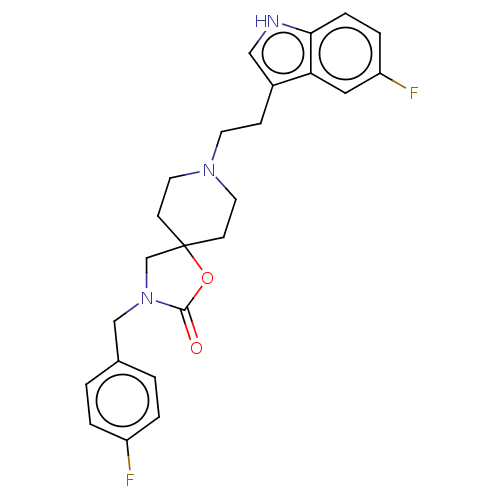
(CHEMBL122169)Show SMILES Fc1ccc(CN2CC3(CCN(CCc4c[nH]c5ccc(F)cc45)CC3)OC2=O)cc1 Show InChI InChI=1S/C24H25F2N3O2/c25-19-3-1-17(2-4-19)15-29-16-24(31-23(29)30)8-11-28(12-9-24)10-7-18-14-27-22-6-5-20(26)13-21(18)22/h1-6,13-14,27H,7-12,15-16H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470603
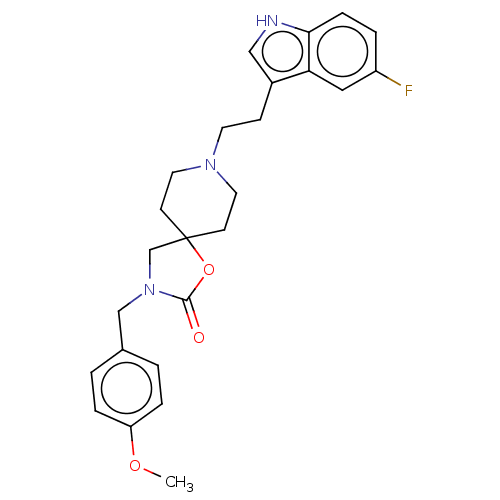
(CHEMBL124013)Show SMILES COc1ccc(CN2CC3(CCN(CCc4c[nH]c5ccc(F)cc45)CC3)OC2=O)cc1 Show InChI InChI=1S/C25H28FN3O3/c1-31-21-5-2-18(3-6-21)16-29-17-25(32-24(29)30)9-12-28(13-10-25)11-8-19-15-27-23-7-4-20(26)14-22(19)23/h2-7,14-15,27H,8-13,16-17H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470582

(CHEMBL126043)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CC2(CCN(CCc3c[nH]c4ccc(F)cc34)CC2)OC1=O Show InChI InChI=1S/C24H26FN3O4S/c1-17-2-5-20(6-3-17)33(30,31)28-16-24(32-23(28)29)9-12-27(13-10-24)11-8-18-15-26-22-7-4-19(25)14-21(18)22/h2-7,14-15,26H,8-13,16H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470599
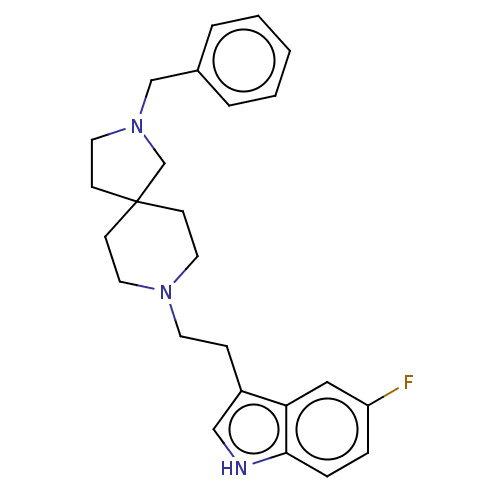
(CHEMBL330766)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CCN(Cc5ccccc5)C4)CC3)c2c1 Show InChI InChI=1S/C25H30FN3/c26-22-6-7-24-23(16-22)21(17-27-24)8-12-28-13-9-25(10-14-28)11-15-29(19-25)18-20-4-2-1-3-5-20/h1-7,16-17,27H,8-15,18-19H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470595
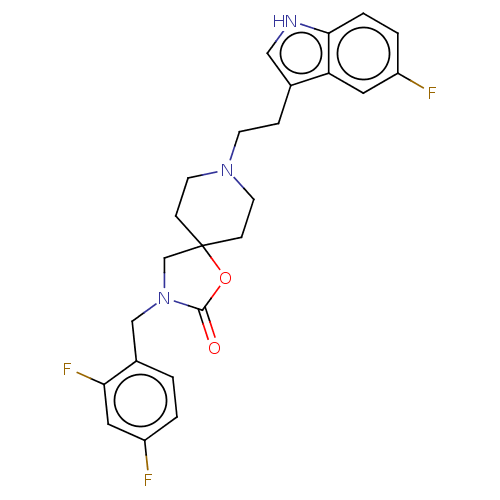
(CHEMBL122019)Show SMILES Fc1ccc(CN2CC3(CCN(CCc4c[nH]c5ccc(F)cc45)CC3)OC2=O)c(F)c1 Show InChI InChI=1S/C24H24F3N3O2/c25-18-3-4-22-20(11-18)16(13-28-22)5-8-29-9-6-24(7-10-29)15-30(23(31)32-24)14-17-1-2-19(26)12-21(17)27/h1-4,11-13,28H,5-10,14-15H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470592
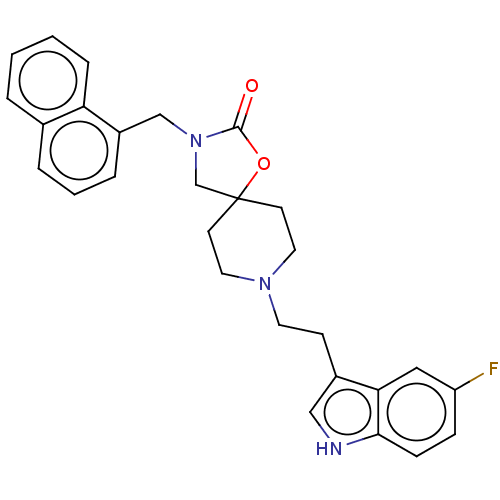
(CHEMBL339311)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5cccc6ccccc56)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C28H28FN3O2/c29-23-8-9-26-25(16-23)21(17-30-26)10-13-31-14-11-28(12-15-31)19-32(27(33)34-28)18-22-6-3-5-20-4-1-2-7-24(20)22/h1-9,16-17,30H,10-15,18-19H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50470591

(CHEMBL124208)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccccc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C24H26FN3O2/c25-20-6-7-22-21(14-20)19(15-26-22)8-11-27-12-9-24(10-13-27)17-28(23(29)30-24)16-18-4-2-1-3-5-18/h1-7,14-15,26H,8-13,16-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against Tachykinin receptor 2 from human expressed in CHO cells |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470597

(CHEMBL330826)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccc(Cl)cc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C24H25ClFN3O2/c25-19-3-1-17(2-4-19)15-29-16-24(31-23(29)30)8-11-28(12-9-24)10-7-18-14-27-22-6-5-20(26)13-21(18)22/h1-6,13-14,27H,7-12,15-16H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470606
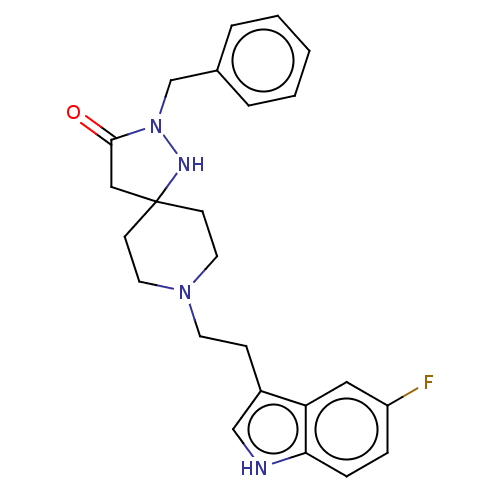
(CHEMBL446099)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CC(=O)N(Cc5ccccc5)N4)CC3)c2c1 Show InChI InChI=1S/C24H27FN4O/c25-20-6-7-22-21(14-20)19(16-26-22)8-11-28-12-9-24(10-13-28)15-23(30)29(27-24)17-18-4-2-1-3-5-18/h1-7,14,16,26-27H,8-13,15,17H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470581

(CHEMBL341008)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(CCc5ccccc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C25H28FN3O2/c26-21-6-7-23-22(16-21)20(17-27-23)9-12-28-14-10-25(11-15-28)18-29(24(30)31-25)13-8-19-4-2-1-3-5-19/h1-7,16-17,27H,8-15,18H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470584
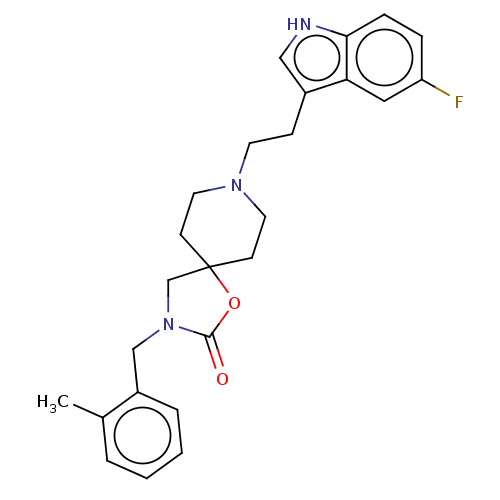
(CHEMBL125001)Show SMILES Cc1ccccc1CN1CC2(CCN(CCc3c[nH]c4ccc(F)cc34)CC2)OC1=O Show InChI InChI=1S/C25H28FN3O2/c1-18-4-2-3-5-20(18)16-29-17-25(31-24(29)30)9-12-28(13-10-25)11-8-19-15-27-23-7-6-21(26)14-22(19)23/h2-7,14-15,27H,8-13,16-17H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470585
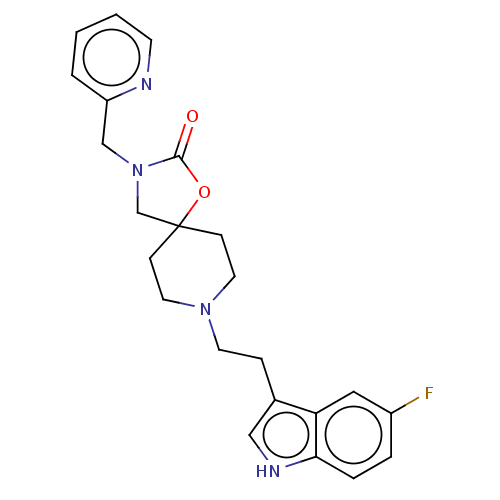
(CHEMBL415518)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccccn5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C23H25FN4O2/c24-18-4-5-21-20(13-18)17(14-26-21)6-10-27-11-7-23(8-12-27)16-28(22(29)30-23)15-19-3-1-2-9-25-19/h1-5,9,13-14,26H,6-8,10-12,15-16H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470601

(CHEMBL123731)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(C(c5ccccc5)c5ccccc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C30H30FN3O2/c31-25-11-12-27-26(19-25)24(20-32-27)13-16-33-17-14-30(15-18-33)21-34(29(35)36-30)28(22-7-3-1-4-8-22)23-9-5-2-6-10-23/h1-12,19-20,28,32H,13-18,21H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470607
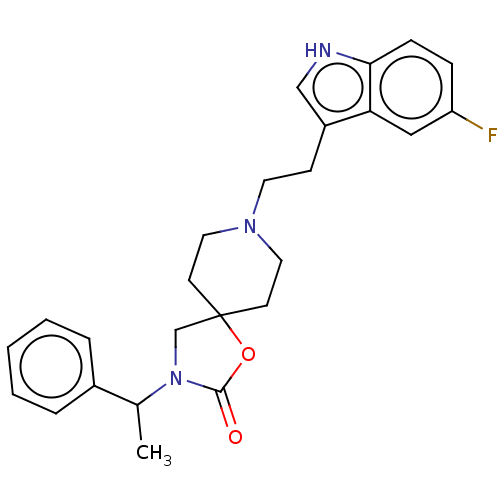
(CHEMBL124624)Show SMILES CC(N1CC2(CCN(CCc3c[nH]c4ccc(F)cc34)CC2)OC1=O)c1ccccc1 Show InChI InChI=1S/C25H28FN3O2/c1-18(19-5-3-2-4-6-19)29-17-25(31-24(29)30)10-13-28(14-11-25)12-9-20-16-27-23-8-7-21(26)15-22(20)23/h2-8,15-16,18,27H,9-14,17H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470608

(CHEMBL338030)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccc6ccccc6c5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C28H28FN3O2/c29-24-7-8-26-25(16-24)23(17-30-26)9-12-31-13-10-28(11-14-31)19-32(27(33)34-28)18-20-5-6-21-3-1-2-4-22(21)15-20/h1-8,15-17,30H,9-14,18-19H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470589

(CHEMBL331011)Show SMILES COC(=O)c1cccc(CN2CC3(CCN(CCc4c[nH]c5ccc(F)cc45)CC3)OC2=O)c1 Show InChI InChI=1S/C26H28FN3O4/c1-33-24(31)19-4-2-3-18(13-19)16-30-17-26(34-25(30)32)8-11-29(12-9-26)10-7-20-15-28-23-6-5-21(27)14-22(20)23/h2-6,13-15,28H,7-12,16-17H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470602

(CHEMBL340747)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccc(cc5)C#N)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C25H25FN4O2/c26-21-5-6-23-22(13-21)20(15-28-23)7-10-29-11-8-25(9-12-29)17-30(24(31)32-25)16-19-3-1-18(14-27)2-4-19/h1-6,13,15,28H,7-12,16-17H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470600

(CHEMBL435619)Show SMILES OC1(CNCc2ccccc2)CCN(CCc2c[nH]c3ccc(F)cc23)CC1 Show InChI InChI=1S/C23H28FN3O/c24-20-6-7-22-21(14-20)19(16-26-22)8-11-27-12-9-23(28,10-13-27)17-25-15-18-4-2-1-3-5-18/h1-7,14,16,25-26,28H,8-13,15,17H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470594
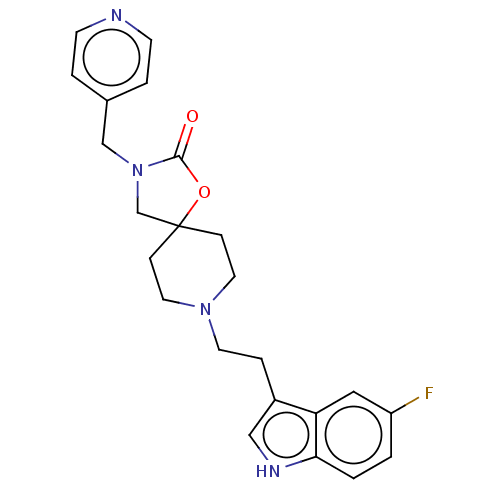
(CHEMBL340405)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccncc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C23H25FN4O2/c24-19-1-2-21-20(13-19)18(14-26-21)5-10-27-11-6-23(7-12-27)16-28(22(29)30-23)15-17-3-8-25-9-4-17/h1-4,8-9,13-14,26H,5-7,10-12,15-16H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470586

(CHEMBL435610)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CC3)NC(=O)N(Cc3ccccc3)C4=O)c2c1 Show InChI InChI=1S/C24H25FN4O2/c25-19-6-7-21-20(14-19)18(15-26-21)8-11-28-12-9-24(10-13-28)22(30)29(23(31)27-24)16-17-4-2-1-3-5-17/h1-7,14-15,26H,8-13,16H2,(H,27,31) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470593
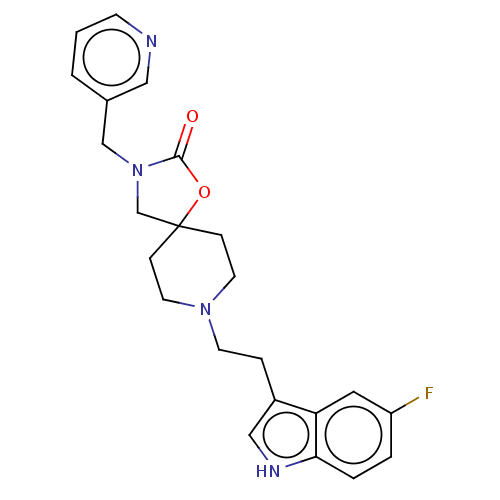
(CHEMBL126158)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5cccnc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C23H25FN4O2/c24-19-3-4-21-20(12-19)18(14-26-21)5-9-27-10-6-23(7-11-27)16-28(22(29)30-23)15-17-2-1-8-25-13-17/h1-4,8,12-14,26H,5-7,9-11,15-16H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50470588
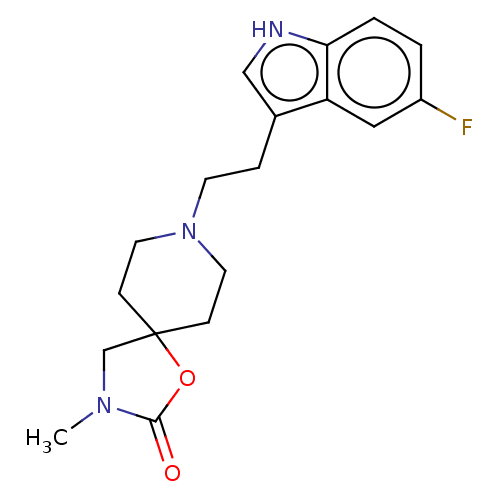
(CHEMBL340548)Show InChI InChI=1S/C18H22FN3O2/c1-21-12-18(24-17(21)23)5-8-22(9-6-18)7-4-13-11-20-16-3-2-14(19)10-15(13)16/h2-3,10-11,20H,4-9,12H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity against tachykinin receptor 2 from rat colon. |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470591

(CHEMBL124208)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccccc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C24H26FN3O2/c25-20-6-7-22-21(14-20)19(15-26-22)8-11-27-12-9-24(10-13-27)17-28(23(29)30-24)16-18-4-2-1-3-5-18/h1-7,14-15,26H,8-13,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against Tachykinin receptor 1 from rabbit cortex |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50470591

(CHEMBL124208)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccccc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C24H26FN3O2/c25-20-6-7-22-21(14-20)19(15-26-22)8-11-27-12-9-24(10-13-27)17-28(23(29)30-24)16-18-4-2-1-3-5-18/h1-7,14-15,26H,8-13,16-17H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against Tachykinin receptor 1 from guinea pig trachea |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM50470591

(CHEMBL124208)Show SMILES Fc1ccc2[nH]cc(CCN3CCC4(CN(Cc5ccccc5)C(=O)O4)CC3)c2c1 Show InChI InChI=1S/C24H26FN3O2/c25-20-6-7-22-21(14-20)19(15-26-22)8-11-27-12-9-24(10-13-27)17-28(23(29)30-24)16-18-4-2-1-3-5-18/h1-7,14-15,26H,8-13,16-17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against Tachykinin receptor 3 from guinea pig cerebral cortex |
J Med Chem 38: 3772-9 (1995)
Article DOI: 10.1021/jm00019a006
BindingDB Entry DOI: 10.7270/Q2JM2DCF |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413296
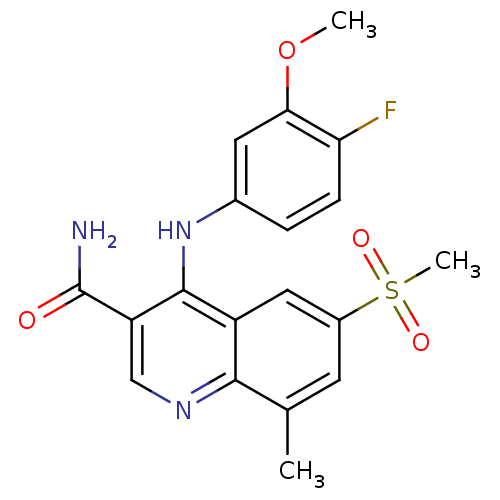
(CHEMBL473764)Show SMILES COc1cc(Nc2c(cnc3c(C)cc(cc23)S(C)(=O)=O)C(N)=O)ccc1F Show InChI InChI=1S/C19H18FN3O4S/c1-10-6-12(28(3,25)26)8-13-17(10)22-9-14(19(21)24)18(13)23-11-4-5-15(20)16(7-11)27-2/h4-9H,1-3H3,(H2,21,24)(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413297
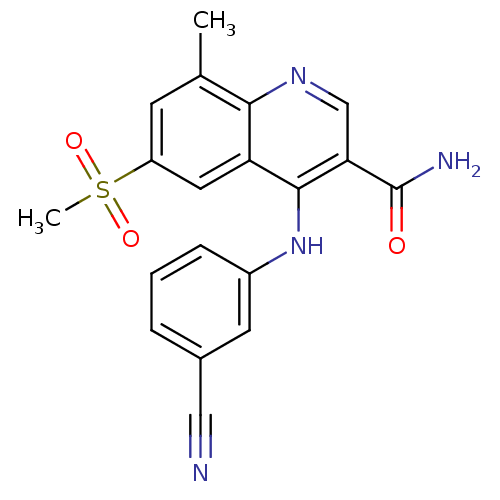
(CHEMBL473560)Show SMILES Cc1cc(cc2c(Nc3cccc(c3)C#N)c(cnc12)C(N)=O)S(C)(=O)=O Show InChI InChI=1S/C19H16N4O3S/c1-11-6-14(27(2,25)26)8-15-17(11)22-10-16(19(21)24)18(15)23-13-5-3-4-12(7-13)9-20/h3-8,10H,1-2H3,(H2,21,24)(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413291
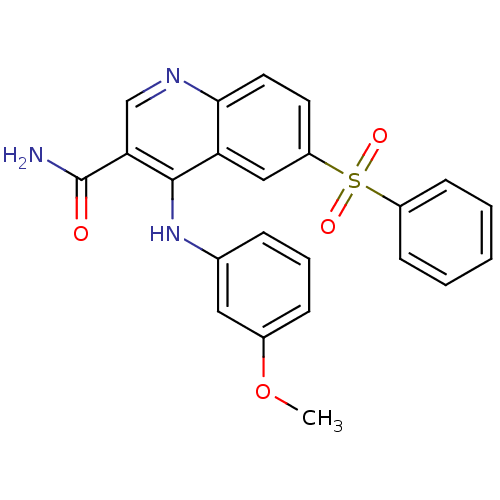
(CHEMBL515240)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2ccccc2)C(N)=O)c1 Show InChI InChI=1S/C23H19N3O4S/c1-30-16-7-5-6-15(12-16)26-22-19-13-18(31(28,29)17-8-3-2-4-9-17)10-11-21(19)25-14-20(22)23(24)27/h2-14H,1H3,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413286
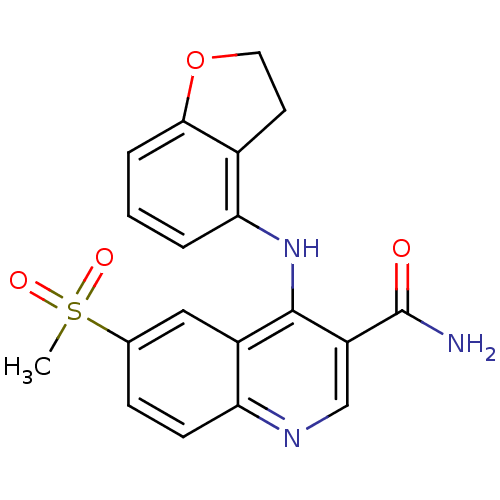
(CHEMBL517766)Show SMILES CS(=O)(=O)c1ccc2ncc(C(N)=O)c(Nc3cccc4OCCc34)c2c1 Show InChI InChI=1S/C19H17N3O4S/c1-27(24,25)11-5-6-15-13(9-11)18(14(10-21-15)19(20)23)22-16-3-2-4-17-12(16)7-8-26-17/h2-6,9-10H,7-8H2,1H3,(H2,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50412414

(CHEMBL521203)Show SMILES CCn1ncc2c(NC3CCOCC3)c(cnc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C21H25N5O2/c1-2-26-20-17(14-24-26)19(25-16-8-10-28-11-9-16)18(13-22-20)21(27)23-12-15-6-4-3-5-7-15/h3-7,13-14,16H,2,8-12H2,1H3,(H,22,25)(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B in Sf9 cells |
Bioorg Med Chem Lett 18: 4237-41 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.052
BindingDB Entry DOI: 10.7270/Q2ZP47B3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413298
(CHEMBL462150)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(C)(=O)=O)C(N)=O)c1 Show InChI InChI=1S/C18H17N3O4S/c1-25-12-5-3-4-11(8-12)21-17-14-9-13(26(2,23)24)6-7-16(14)20-10-15(17)18(19)22/h3-10H,1-2H3,(H2,19,22)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
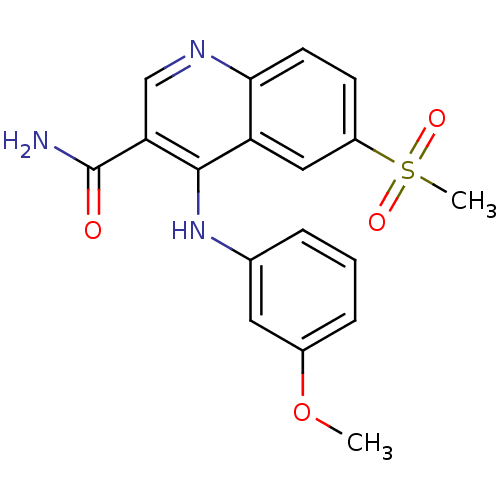
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50521218

(CHEMBL4434674)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1cc(-c2ncc(CN3C[C@H](C)O[C@H](C)C3)o2)c2cn[nH]c2c1 |r| Show InChI InChI=1S/C24H28N6O5S/c1-14-11-30(12-15(2)34-14)13-18-9-26-23(35-18)19-5-16(6-21-20(19)10-27-28-21)17-7-22(29-36(4,31)32)24(33-3)25-8-17/h5-10,14-15,29H,11-13H2,1-4H3,(H,27,28)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human PBMC assessed as reduction in cytostim-induced IFNgamma production after 20 hrs by electrochemiluminescence assay |
J Med Chem 59: 7239-51 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00799
BindingDB Entry DOI: 10.7270/Q26M3BBF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50536411
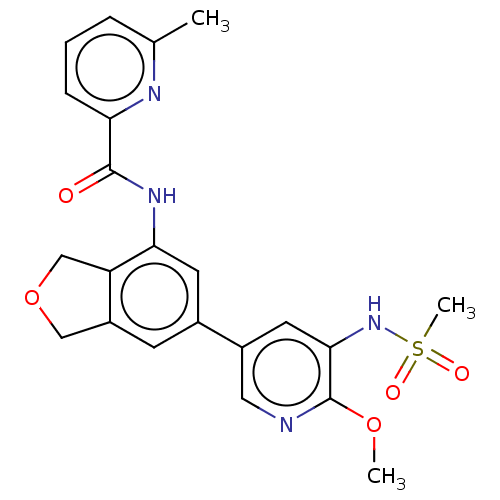
(CHEMBL4564931)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1cc2COCc2c(NC(=O)c2cccc(C)n2)c1 Show InChI InChI=1S/C22H22N4O5S/c1-13-5-4-6-18(24-13)21(27)25-19-8-14(7-16-11-31-12-17(16)19)15-9-20(26-32(3,28)29)22(30-2)23-10-15/h4-10,26H,11-12H2,1-3H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 59: 7239-51 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00799
BindingDB Entry DOI: 10.7270/Q26M3BBF |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413306
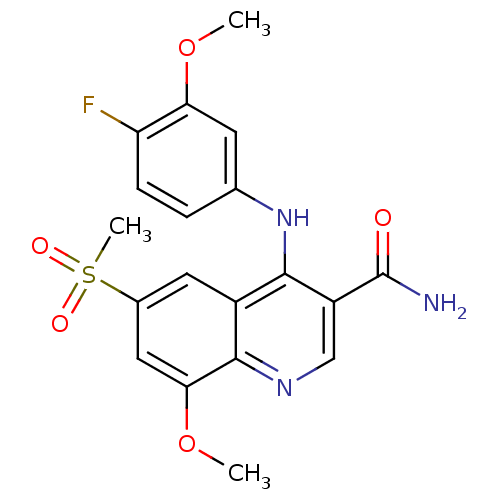
(CHEMBL515098)Show SMILES COc1cc(Nc2c(cnc3c(OC)cc(cc23)S(C)(=O)=O)C(N)=O)ccc1F Show InChI InChI=1S/C19H18FN3O5S/c1-27-15-6-10(4-5-14(15)20)23-17-12-7-11(29(3,25)26)8-16(28-2)18(12)22-9-13(17)19(21)24/h4-9H,1-3H3,(H2,21,24)(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50521215
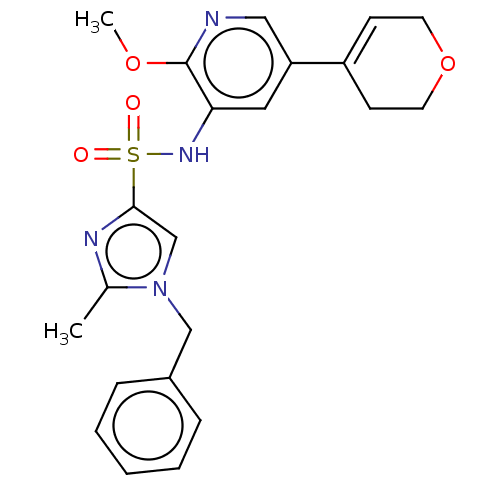
(CHEMBL4435246)Show SMILES COc1ncc(cc1NS(=O)(=O)c1cn(Cc2ccccc2)c(C)n1)C1=CCOCC1 |t:28| Show InChI InChI=1S/C22H24N4O4S/c1-16-24-21(15-26(16)14-17-6-4-3-5-7-17)31(27,28)25-20-12-19(13-23-22(20)29-2)18-8-10-30-11-9-18/h3-8,12-13,15,25H,9-11,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins by HT... |
J Med Chem 61: 11061-11073 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01556
BindingDB Entry DOI: 10.7270/Q2Z60SFQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413288
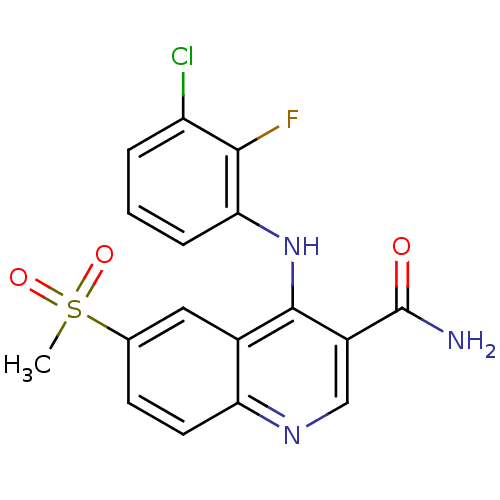
(CHEMBL517590)Show SMILES CS(=O)(=O)c1ccc2ncc(C(N)=O)c(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C17H13ClFN3O3S/c1-26(24,25)9-5-6-13-10(7-9)16(11(8-21-13)17(20)23)22-14-4-2-3-12(18)15(14)19/h2-8H,1H3,(H2,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50536416

(CHEMBL4522825)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1cc2COCc2c(NC(=O)c2csc(C)n2)c1 Show InChI InChI=1S/C20H20N4O5S2/c1-11-22-18(10-30-11)19(25)23-16-5-12(4-14-8-29-9-15(14)16)13-6-17(24-31(3,26)27)20(28-2)21-7-13/h4-7,10,24H,8-9H2,1-3H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 59: 7239-51 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00799
BindingDB Entry DOI: 10.7270/Q26M3BBF |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50412405

(CHEMBL488634)Show SMILES CCn1ncc2c(NC3CCOCC3)c(cnc12)C(=O)NCC1CCCCC1 Show InChI InChI=1S/C21H31N5O2/c1-2-26-20-17(14-24-26)19(25-16-8-10-28-11-9-16)18(13-22-20)21(27)23-12-15-6-4-3-5-7-15/h13-16H,2-12H2,1H3,(H,22,25)(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B in Sf9 cells |
Bioorg Med Chem Lett 18: 4237-41 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.052
BindingDB Entry DOI: 10.7270/Q2ZP47B3 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase LCK
(Mus musculus) | BDBM26166
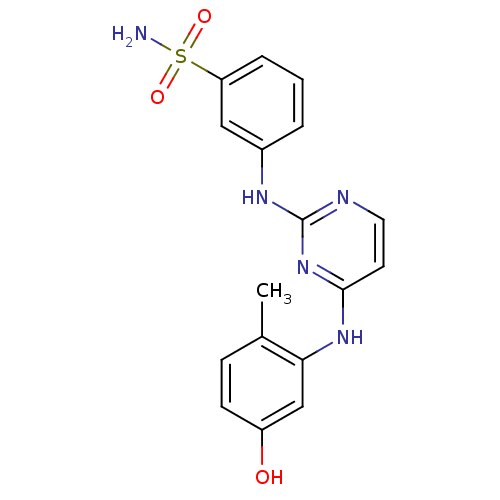
(2,4-dianilino pyrimidine, 23 | 3-({4-[(5-hydroxy-2...)Show SMILES Cc1ccc(O)cc1Nc1ccnc(Nc2cccc(c2)S(N)(=O)=O)n1 Show InChI InChI=1S/C17H17N5O3S/c1-11-5-6-13(23)10-15(11)21-16-7-8-19-17(22-16)20-12-3-2-4-14(9-12)26(18,24)25/h2-10,23H,1H3,(H2,18,24,25)(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK
| Assay Description
Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... |
Bioorg Med Chem Lett 17: 4363-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.029
BindingDB Entry DOI: 10.7270/Q21R6NTD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data