

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50002515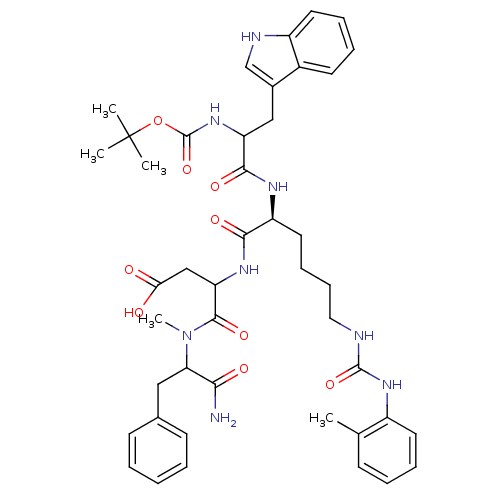 ((A-71623)3-[2-[2-tert-Butoxycarbonylamino-3-(1H-in...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM81870 (A-70874 | CAS_6439313 | NSC_6439313) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM81869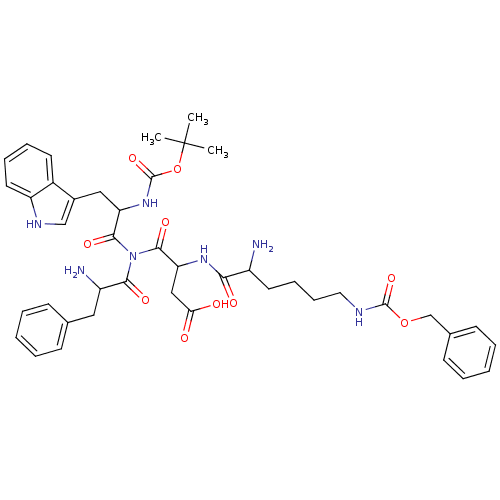 (A-57282) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM82558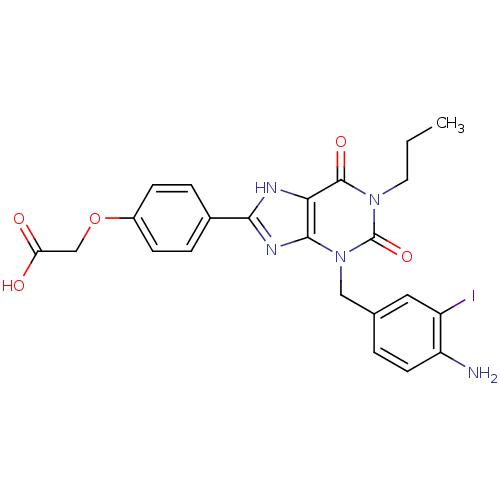 (CAS_163889 | CHEMBL355370 | I-ABOPX | NSC_163889) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor of rat brain membranes without NaCl by inhibition of [125-I]-labeled aminobenzyl adenosine binding | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM81870 (A-70874 | CAS_6439313 | NSC_6439313) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50008405 (2-(4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023392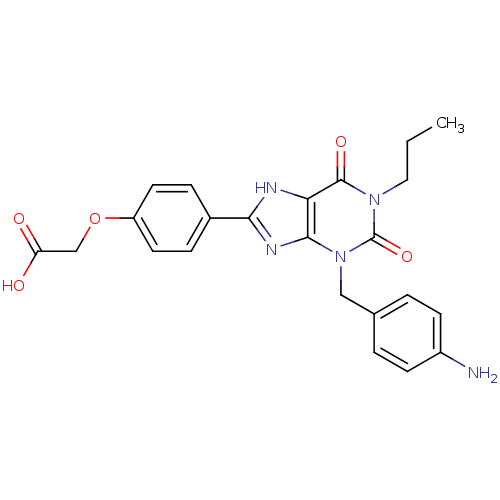 (CHEMBL349519 | {4-[3-(4-Amino-benzyl)-2,6-dioxo-1-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023391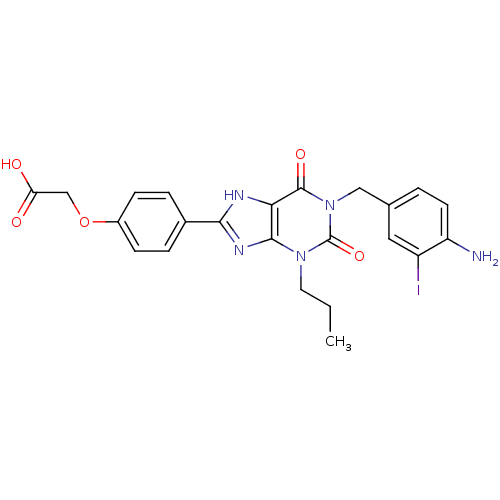 (CHEMBL162814 | {4-[1-(4-Amino-3-iodo-benzyl)-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023399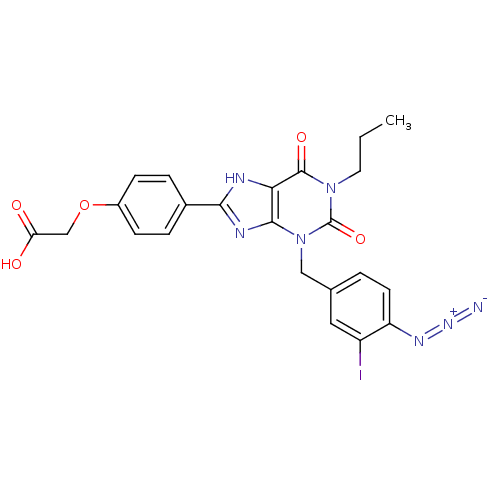 (CHEMBL162658 | {4-[3-(4-Azido-3-iodo-benzyl)-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50002515 ((A-71623)3-[2-[2-tert-Butoxycarbonylamino-3-(1H-in...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023398 ((4-{3-[2-(4-Amino-3-iodo-phenyl)-ethyl]-2,6-dioxo-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023397 (CHEMBL162558 | {4-[1-(4-Azido-3-iodo-benzyl)-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023395 ((4-{3-[2-(4-Azido-3-iodo-phenyl)-ethyl]-2,6-dioxo-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023401 ((4-{1-[2-(4-Amino-phenyl)-ethyl]-2,6-dioxo-3-propy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor of rat brain membranes without NaCl by inhibition of [125-I]-labeled aminobenzyl adenosine binding | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023396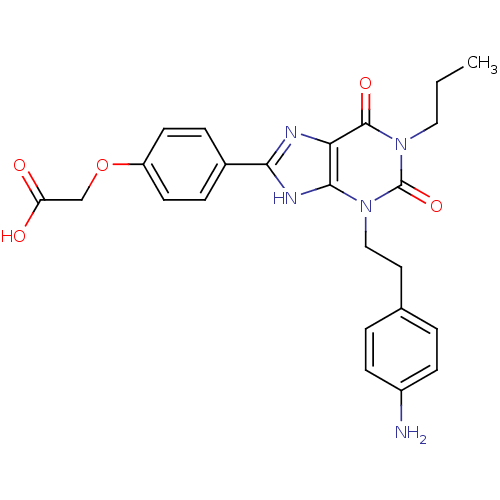 ((4-{3-[2-(4-Amino-phenyl)-ethyl]-2,6-dioxo-1-propy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023394 ((4-{1-[2-(4-Azido-3-iodo-phenyl)-ethyl]-2,6-dioxo-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023402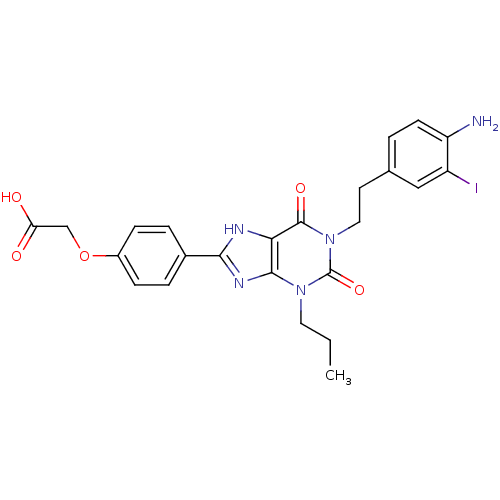 ((4-{1-[2-(4-Amino-3-iodo-phenyl)-ethyl]-2,6-dioxo-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023390 (CHEMBL163574 | {4-[1-(4-Amino-benzyl)-2,6-dioxo-3-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50023400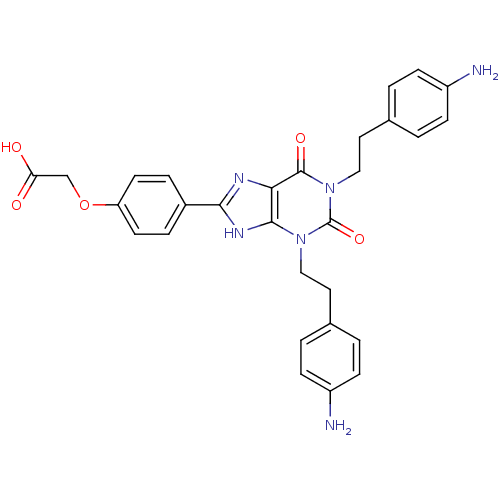 ((4-{1,3-Bis-[2-(4-amino-phenyl)-ethyl]-2,6-dioxo-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Inhibition of NECA binding to adenosine A2 receptor mediates reduced adenylate cyclase activity in human platelets | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor of rat brain membranes without NaCl by inhibition of [125-I]-labeled aminobenzyl adenosine binding | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50367678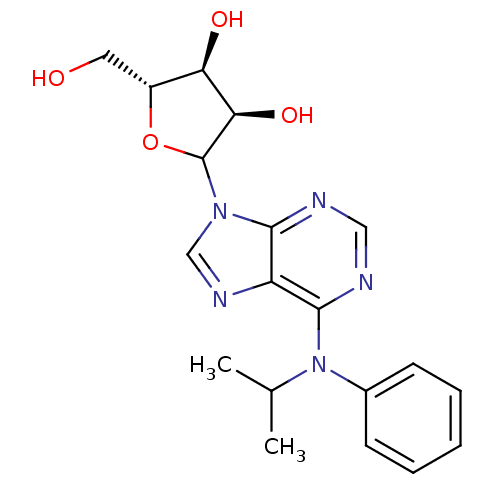 (CHEMBL605469) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor of rat brain membranes without NaCl by inhibition of [125-I]-labeled aminobenzyl adenosine binding | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044039 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044033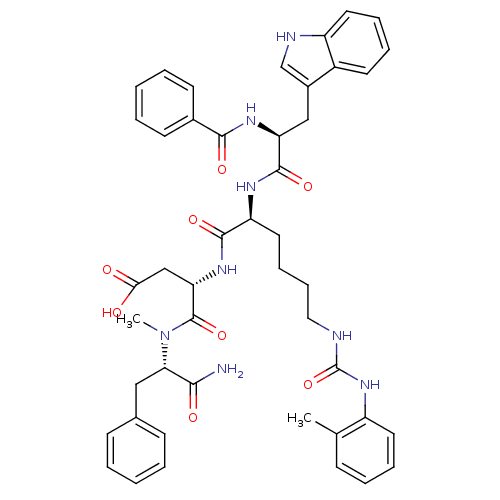 ((S)-3-[(S)-2-((S)-2-Benzoylamino-3-1H-indol-3-yl-p...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044042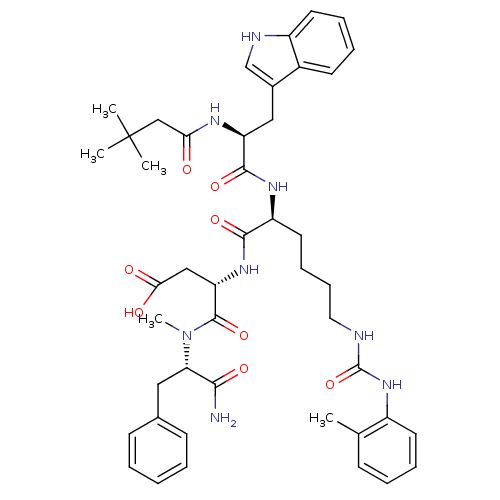 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[2...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044028 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-((...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044038 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50609716 (CHEMBL5272522) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044044 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-((...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 13 (Homo sapiens (Human)) | BDBM50609715 (CHEMBL5286166) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM289504 (1-(4-(6-amino-5-(tri- fluoromethyl)pyridin-3- yl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50609715 (CHEMBL5286166) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044032 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044037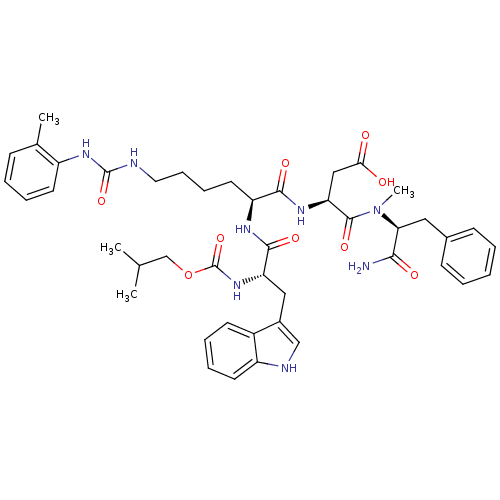 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-((...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor of rat brain membranes without NaCl by inhibition of [125-I]-labeled aminobenzyl adenosine binding | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50002514 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]BH-CCK-8 in guinea pig pancreas. | J Med Chem 35: 2007-14 (1992) BindingDB Entry DOI: 10.7270/Q21J9BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 13 (Homo sapiens (Human)) | BDBM50059190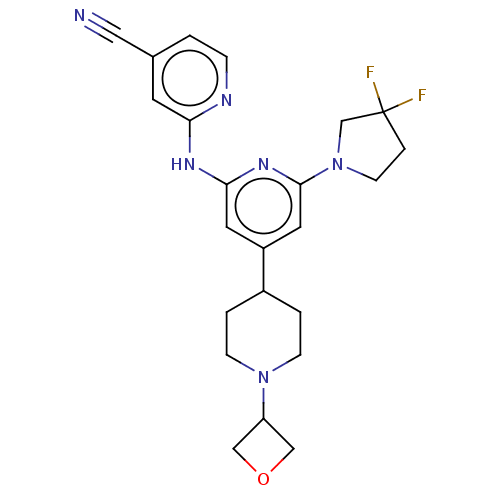 (CHEMBL3393333) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50059190 (CHEMBL3393333) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50609719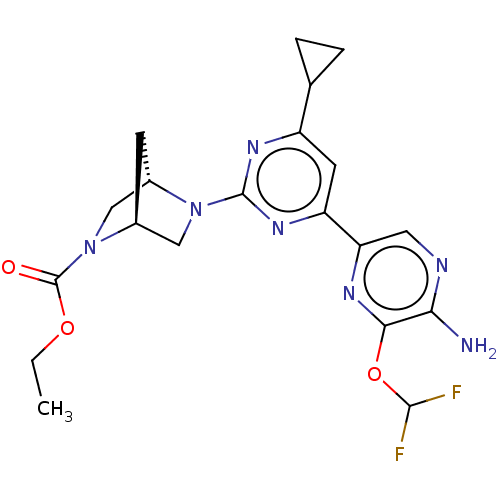 (CHEMBL5283101) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50609718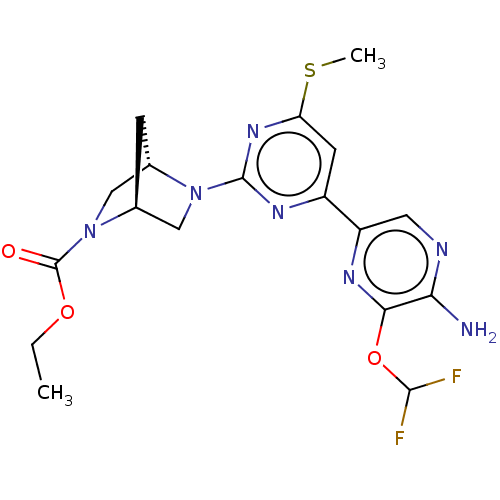 (CHEMBL5288706) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82558 (CAS_163889 | CHEMBL355370 | I-ABOPX | NSC_163889) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor of rat brain membranes with 1 M NaCl by inhibition of [125-I]-labeled aminobenzyl adenosine binding | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50609708 (CHEMBL5281494) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PDB UniChem | PDB | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82558 (CAS_163889 | CHEMBL355370 | I-ABOPX | NSC_163889) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor of rat brain membranes with 1 M NaCl by inhibition of [125-I]-labeled aminobenzyl adenosine binding | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044031 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040523 ((S)-3-{[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50002523 (3-{6-[(6-Acetoxy-naphthalene-2-carbonyl)-amino]-2-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]BH-CCK-8 in guinea pig pancreas. | J Med Chem 35: 2007-14 (1992) BindingDB Entry DOI: 10.7270/Q21J9BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 724 total ) | Next | Last >> |