

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50037287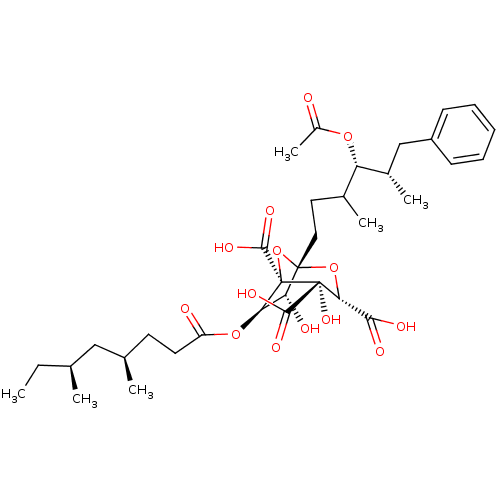 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033185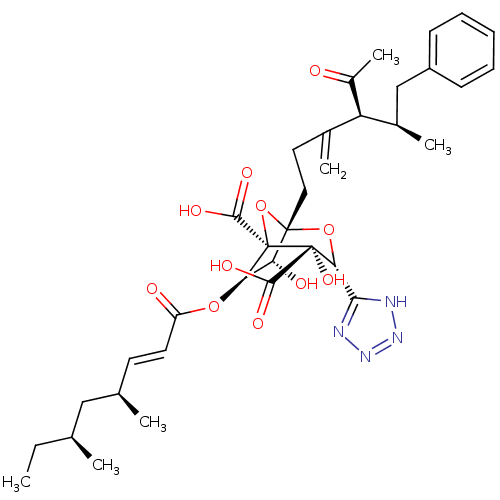 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037286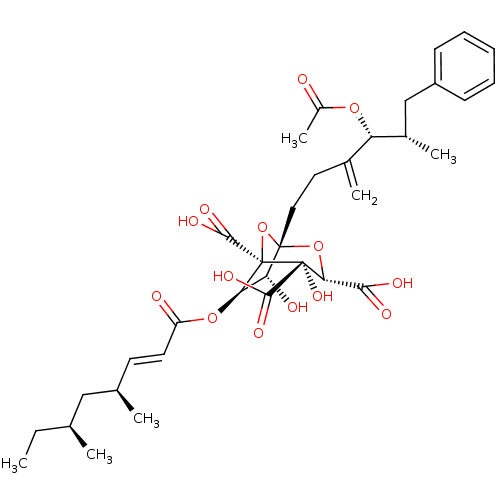 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037285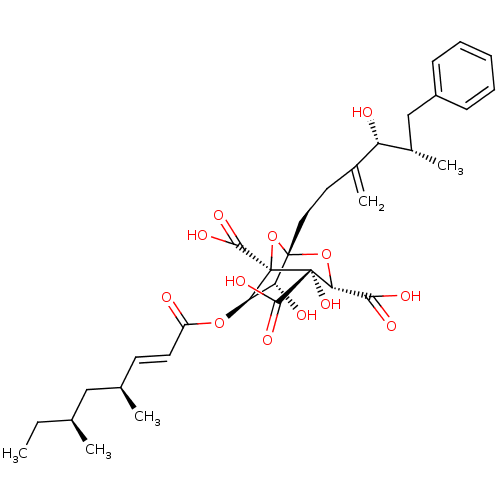 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033196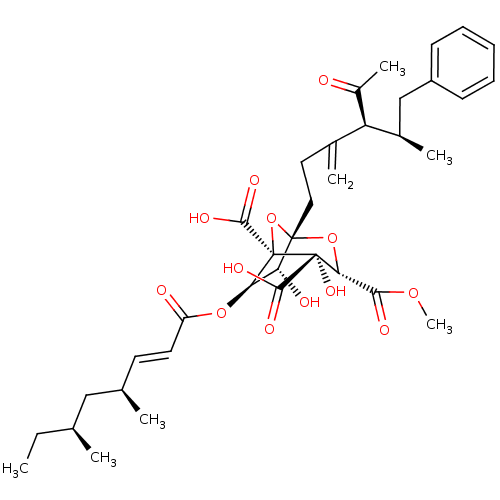 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037279 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037279 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033184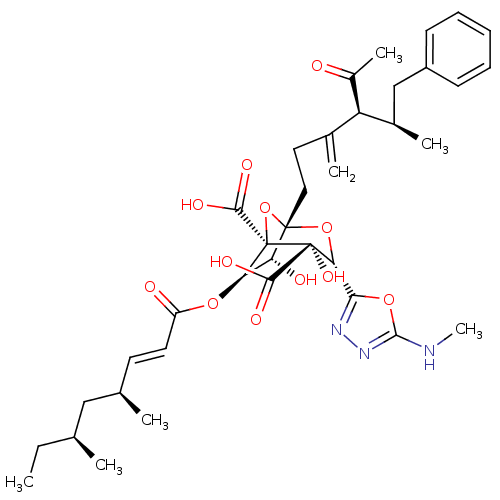 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033199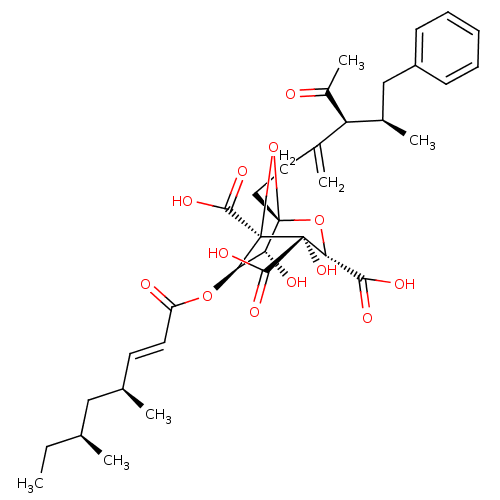 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037286 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037289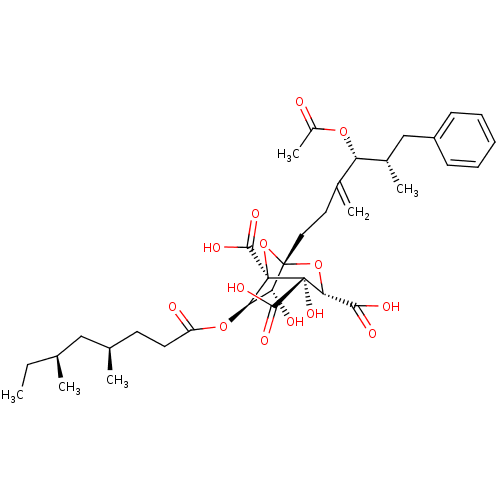 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037291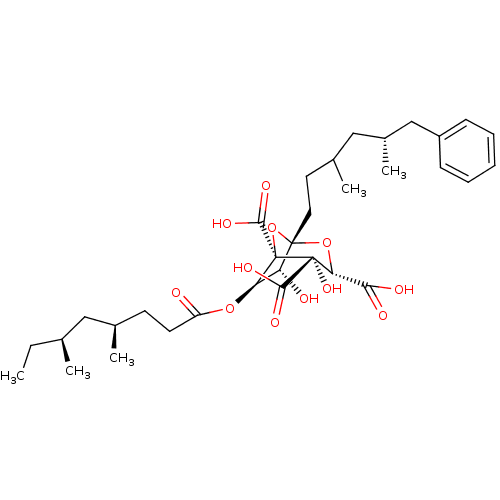 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037291 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078544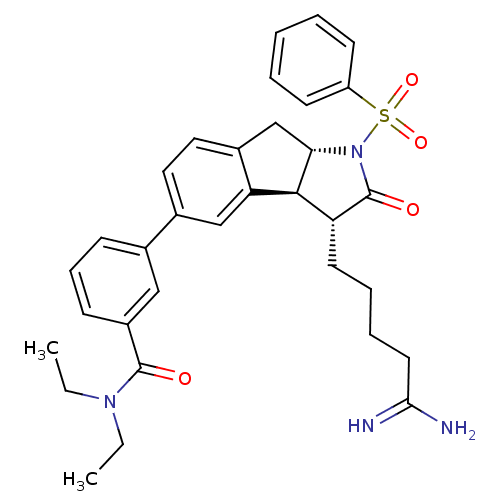 (3-[(3R,3aS,8aS)-1-Benzenesulfonyl-3-(4-carbamimido...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078554 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037290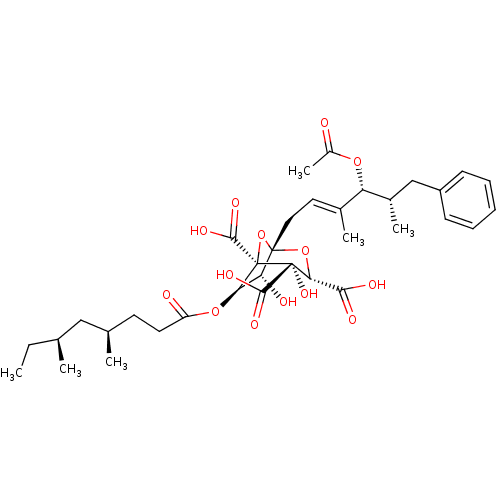 ((1S,3S,4S,5R,6R,7R)-1-((E)-(4R,5S)-4-Acetoxy-3,5-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033187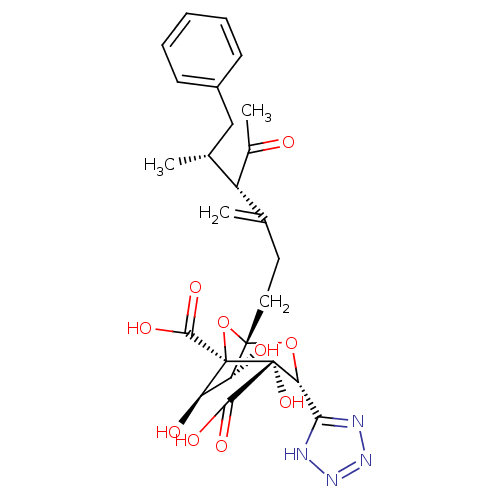 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033181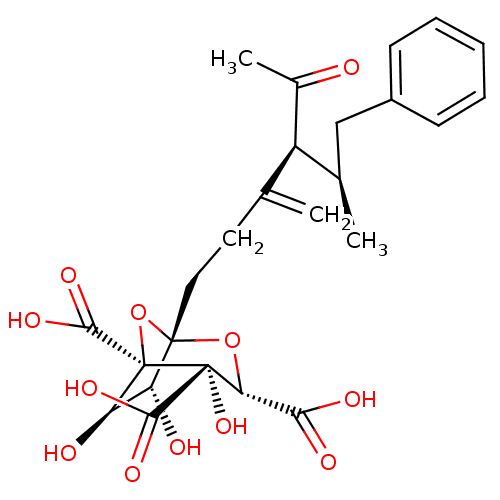 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037280 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078554 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037288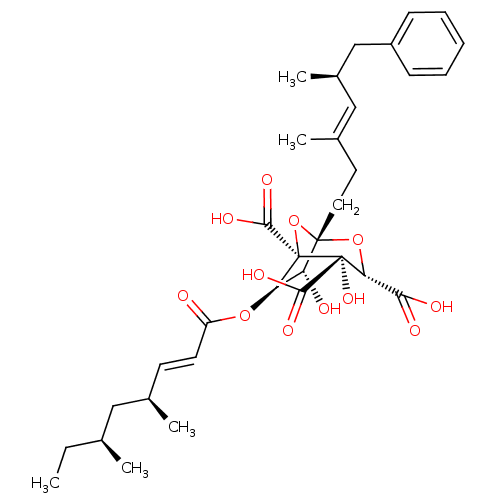 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033192 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078553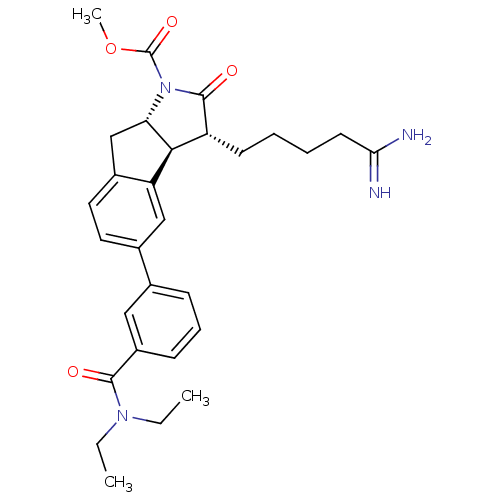 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033205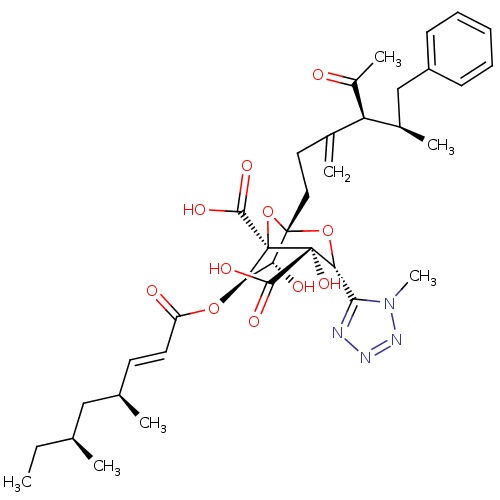 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033186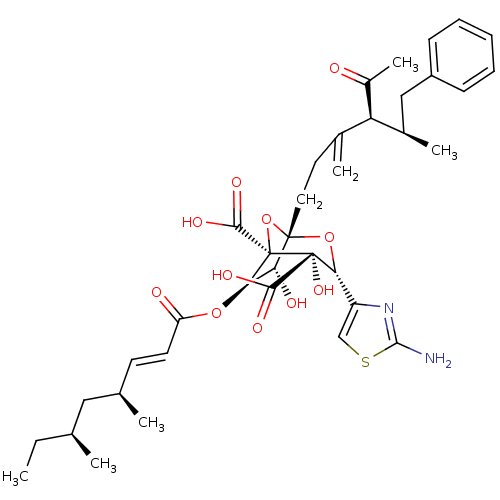 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037282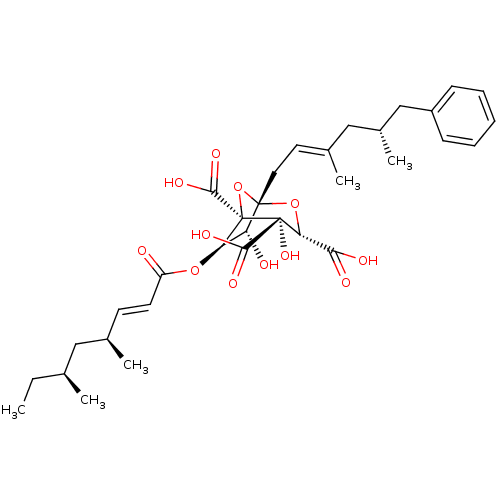 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078544 (3-[(3R,3aS,8aS)-1-Benzenesulfonyl-3-(4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033200 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078544 (3-[(3R,3aS,8aS)-1-Benzenesulfonyl-3-(4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033190 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078554 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078552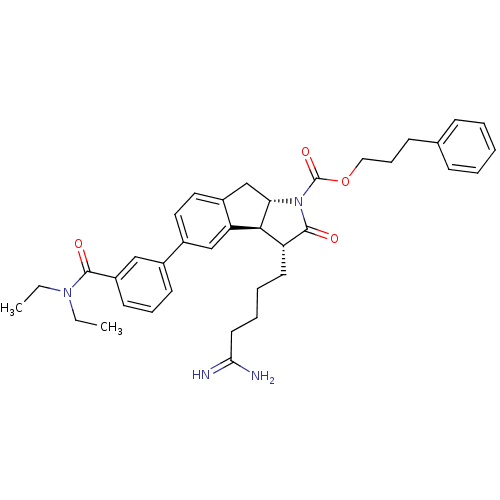 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037284 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033183 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033193 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033195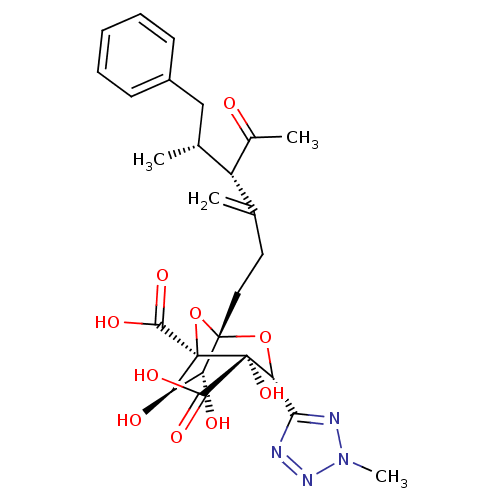 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033182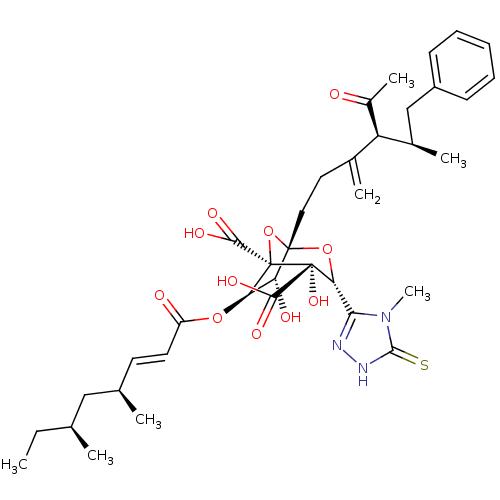 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037283 ((1S,3S,4S,5R,6R,7R)-1-((E)-(S)-3,5-Dimethyl-6-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037281 ((1S,3S,4S,5R,6R,7R)-1-((R)-3,5-Dimethyl-6-phenyl-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037284 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037283 ((1S,3S,4S,5R,6R,7R)-1-((E)-(S)-3,5-Dimethyl-6-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033197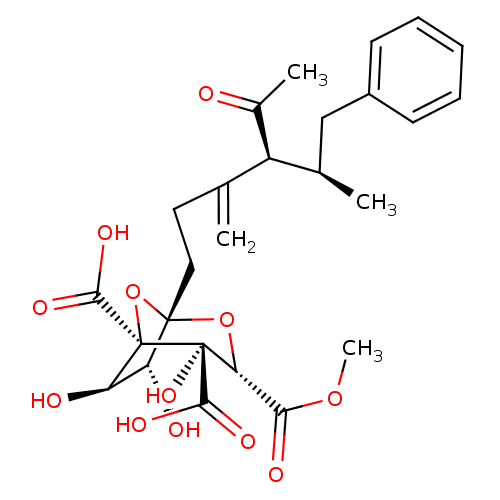 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037292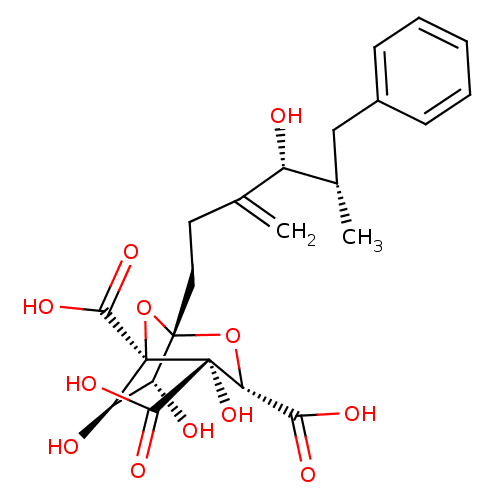 ((1S,3S,4S,5R,6R,7R)-4,6,7-Trihydroxy-1-((4R,5S)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033194 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033198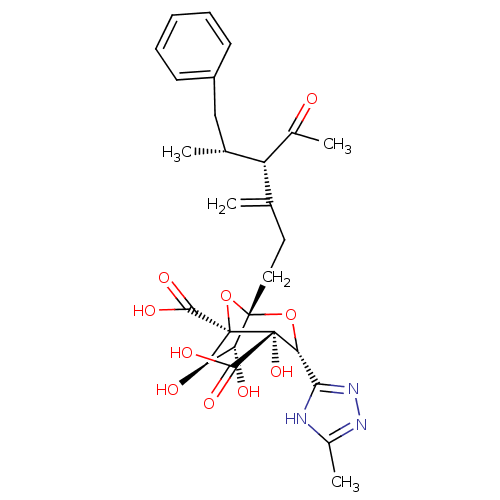 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033204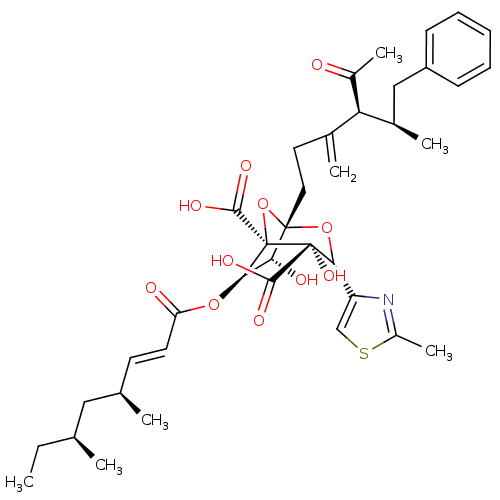 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078556 (3-[(3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-2-oxo-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 97 total ) | Next | Last >> |