

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50172545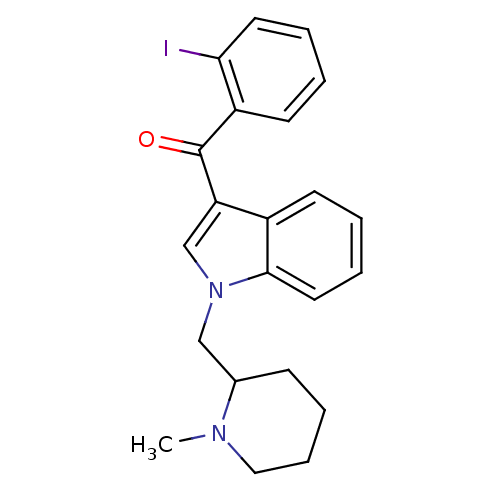 ((2-Iodo-phenyl)-[1-((S)-1-methyl-piperidin-2-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse hippocampal membranes cannabinoid receptor 1 using [131I]-(R)-8 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172553 (CHEMBL68641 | [1-(1-Methyl-piperidin-2-ylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50172545 ((2-Iodo-phenyl)-[1-((S)-1-methyl-piperidin-2-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse hippocampal membranes cannabinoid receptor 1 using [3H]SR-141,716A | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse hippocampal membranes cannabinoid receptor 1 using [131I]-(R)-8 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50172553 (CHEMBL68641 | [1-(1-Methyl-piperidin-2-ylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172545 ((2-Iodo-phenyl)-[1-((S)-1-methyl-piperidin-2-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50172545 ((2-Iodo-phenyl)-[1-((S)-1-methyl-piperidin-2-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50174549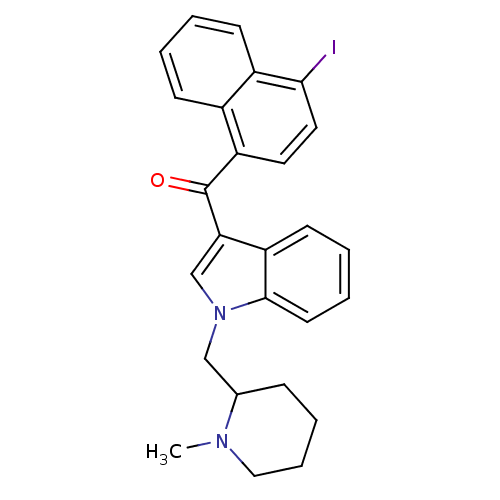 ((4-Iodo-naphthalen-1-yl)-[1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50314706 ((R)-2-(2-naphthamido)-5-(3-fluorophenyl)pent-4-eno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His tagged human Pin1 expressed in Escherichia coli BL21 (DE3) using Suc-Ala-Glu-Pro-Phe-4-nitroanilide as substrate preincu... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115878 BindingDB Entry DOI: 10.7270/Q21J9FDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM34012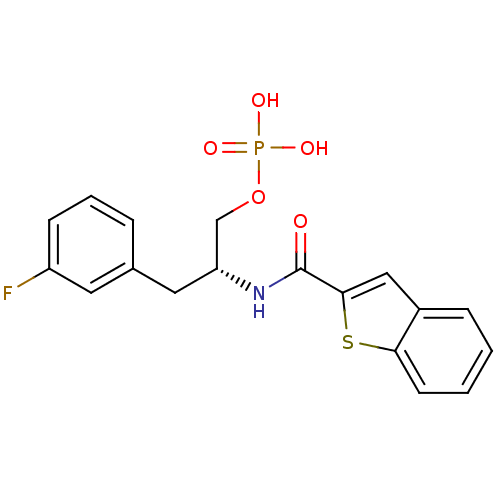 (3-fluorophenylalanine derivative, 21b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Pin1 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmc.2020.115878 BindingDB Entry DOI: 10.7270/Q21J9FDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50174548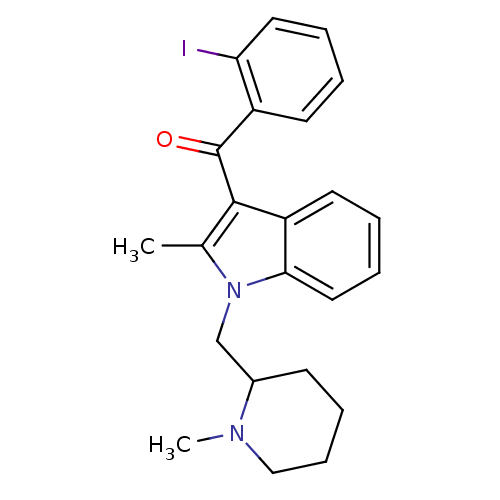 ((2-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50174547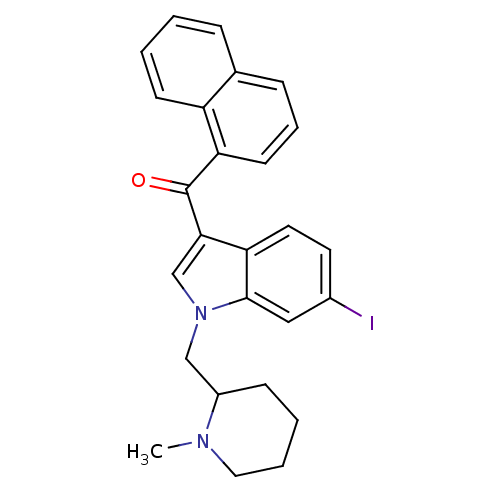 (CHEMBL436120 | [6-Iodo-1-(1-methyl-piperidin-2-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50174549 ((4-Iodo-naphthalen-1-yl)-[1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50174548 ((2-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50174547 (CHEMBL436120 | [6-Iodo-1-(1-methyl-piperidin-2-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50552327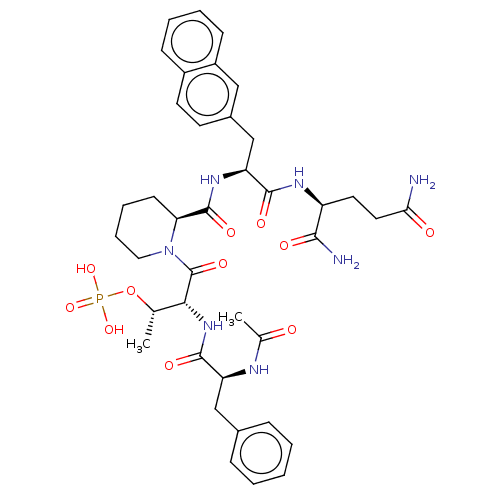 (CHEMBL4751817) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-tagged Pin1 (unknown origin) using Suc-Ala-pSer-Pro-Phe-pNA as substrate preincubated with enzyme for 12 hrs followed by substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115878 BindingDB Entry DOI: 10.7270/Q21J9FDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse hippocampal membranes cannabinoid receptor 1 using [3H]SR-141,716A | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50174548 ((2-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50174548 ((2-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50174548 ((2-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50174548 ((2-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50552328 (CHEMBL4750217) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-tagged Pin1 (unknown origin) using Suc-Ala-pSer-Pro-Phe-pNA as substrate preincubated with enzyme for 12 hrs followed by substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115878 BindingDB Entry DOI: 10.7270/Q21J9FDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50535516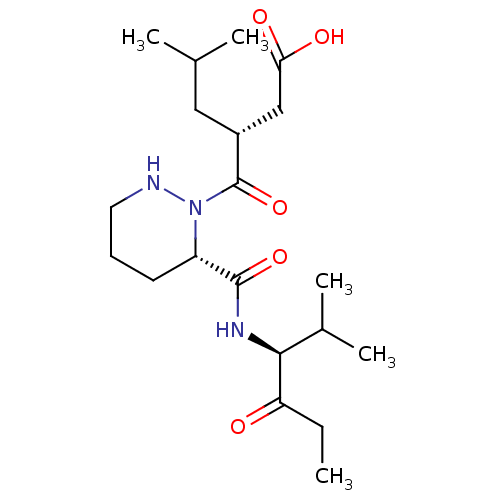 (CHEMBL4542964) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli PDF | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50370681 (CHEMBL1788276) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50370681 (CHEMBL1788276) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50174546 ((4-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM31883 (9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-tagged Pin1 (unknown origin) using Suc-Ala-pSer-Pro-Phe-pNA, Suc-Ala-Glu-Pro-Phe-pNA or Suc-Ala-Ala-Pro-Phe-pNA as substrate preinc... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115878 BindingDB Entry DOI: 10.7270/Q21J9FDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50174546 ((4-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50174545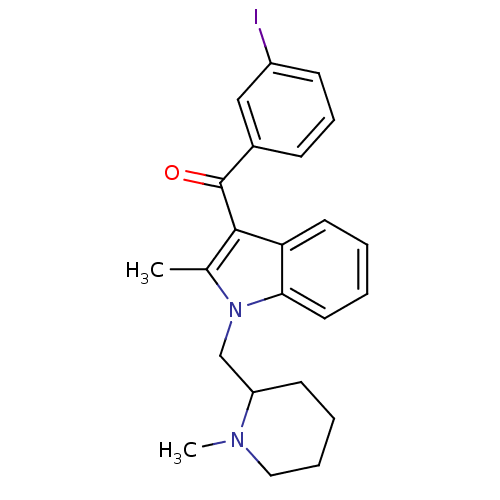 ((3-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50174545 ((3-Iodo-phenyl)-[2-methyl-1-(1-methyl-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity towards mouse spleen cannabinoid receptor 2 using [3H]CP-55940 | J Med Chem 48: 6386-92 (2005) Article DOI: 10.1021/jm050135l BindingDB Entry DOI: 10.7270/Q21V5FQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535514 (CHEMBL4458398) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by absorbance detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535509 (CHEMBL4457234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by absorbance detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50268281 (CHEMBL4067999) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Key Laboratory of Active Substance Discovery and Druggability Evaluation, Institute of Materia Medica, Chinese Academy of Medical Sciences& Peking Union Medical College, Beijing 100050, C Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP-2 expressed in Escherichia coli BL21 (DE3) using sheared DNA as substrate in presence of biotinylated NAD after ... | Bioorg Med Chem 25: 4045-4054 (2017) Article DOI: 10.1016/j.bmc.2017.05.052 BindingDB Entry DOI: 10.7270/Q24Q7XFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535505 (CHEMBL4462876) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by absorbance detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535513 (CHEMBL4572153) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by fluorescence detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50268281 (CHEMBL4067999) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Key Laboratory of Active Substance Discovery and Druggability Evaluation, Institute of Materia Medica, Chinese Academy of Medical Sciences& Peking Union Medical College, Beijing 100050, C Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP-1 expressed in Escherichia coli BL21 (DE3) using sheared DNA as substrate in presence of biotinylated NAD after ... | Bioorg Med Chem 25: 4045-4054 (2017) Article DOI: 10.1016/j.bmc.2017.05.052 BindingDB Entry DOI: 10.7270/Q24Q7XFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM27566 (4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Key Laboratory of Active Substance Discovery and Druggability Evaluation, Institute of Materia Medica, Chinese Academy of Medical Sciences& Peking Union Medical College, Beijing 100050, C Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP-2 expressed in Escherichia coli BL21 (DE3) using sheared DNA as substrate in presence of biotinylated NAD after ... | Bioorg Med Chem 25: 4045-4054 (2017) Article DOI: 10.1016/j.bmc.2017.05.052 BindingDB Entry DOI: 10.7270/Q24Q7XFR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535512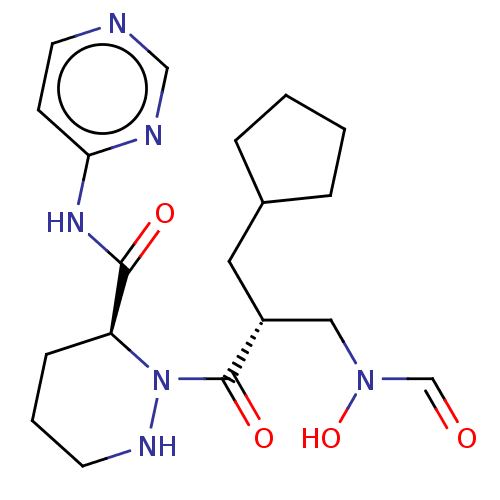 (CHEMBL4434997) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by absorbance detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535508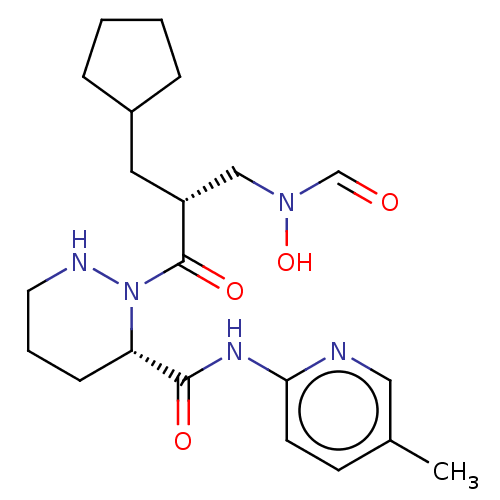 (CHEMBL4518477) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by fluorescence detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535497 (CHEMBL4466084) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by absorbance detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535515 (CHEMBL4559778) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by fluorescence detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535500 (CHEMBL4568739) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by absorbance detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535518 (CHEMBL4448951) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by absorbance detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50268267 (CHEMBL4083291) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Key Laboratory of Active Substance Discovery and Druggability Evaluation, Institute of Materia Medica, Chinese Academy of Medical Sciences& Peking Union Medical College, Beijing 100050, C Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP-2 expressed in Escherichia coli BL21 (DE3) using sheared DNA as substrate in presence of biotinylated NAD after ... | Bioorg Med Chem 25: 4045-4054 (2017) Article DOI: 10.1016/j.bmc.2017.05.052 BindingDB Entry DOI: 10.7270/Q24Q7XFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535504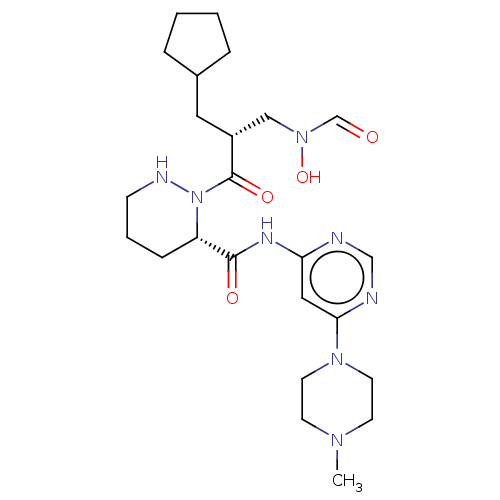 (CHEMBL4565584) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by fluorescence detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50268267 (CHEMBL4083291) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Key Laboratory of Active Substance Discovery and Druggability Evaluation, Institute of Materia Medica, Chinese Academy of Medical Sciences& Peking Union Medical College, Beijing 100050, C Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP-1 expressed in Escherichia coli BL21 (DE3) using sheared DNA as substrate in presence of biotinylated NAD after ... | Bioorg Med Chem 25: 4045-4054 (2017) Article DOI: 10.1016/j.bmc.2017.05.052 BindingDB Entry DOI: 10.7270/Q24Q7XFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50268268 (CHEMBL4061458) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Key Laboratory of Active Substance Discovery and Druggability Evaluation, Institute of Materia Medica, Chinese Academy of Medical Sciences& Peking Union Medical College, Beijing 100050, C Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP-2 expressed in Escherichia coli BL21 (DE3) using sheared DNA as substrate in presence of biotinylated NAD after ... | Bioorg Med Chem 25: 4045-4054 (2017) Article DOI: 10.1016/j.bmc.2017.05.052 BindingDB Entry DOI: 10.7270/Q24Q7XFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50268284 (CHEMBL4100719) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Key Laboratory of Active Substance Discovery and Druggability Evaluation, Institute of Materia Medica, Chinese Academy of Medical Sciences& Peking Union Medical College, Beijing 100050, C Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP-2 expressed in Escherichia coli BL21 (DE3) using sheared DNA as substrate in presence of biotinylated NAD after ... | Bioorg Med Chem 25: 4045-4054 (2017) Article DOI: 10.1016/j.bmc.2017.05.052 BindingDB Entry DOI: 10.7270/Q24Q7XFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50268277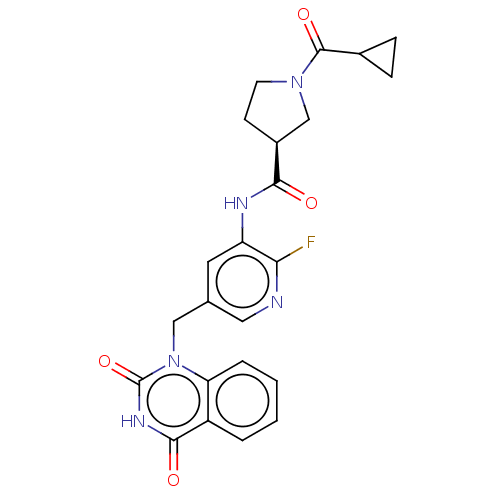 (CHEMBL4072243) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Key Laboratory of Active Substance Discovery and Druggability Evaluation, Institute of Materia Medica, Chinese Academy of Medical Sciences& Peking Union Medical College, Beijing 100050, C Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP-2 expressed in Escherichia coli BL21 (DE3) using sheared DNA as substrate in presence of biotinylated NAD after ... | Bioorg Med Chem 25: 4045-4054 (2017) Article DOI: 10.1016/j.bmc.2017.05.052 BindingDB Entry DOI: 10.7270/Q24Q7XFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50535501 (CHEMBL4435970) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF by absorbance detection assay | Bioorg Med Chem Lett 29: 2410-2414 (2019) Article DOI: 10.1016/j.bmcl.2019.05.028 BindingDB Entry DOI: 10.7270/Q2FF3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 587 total ) | Next | Last >> |