

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50261233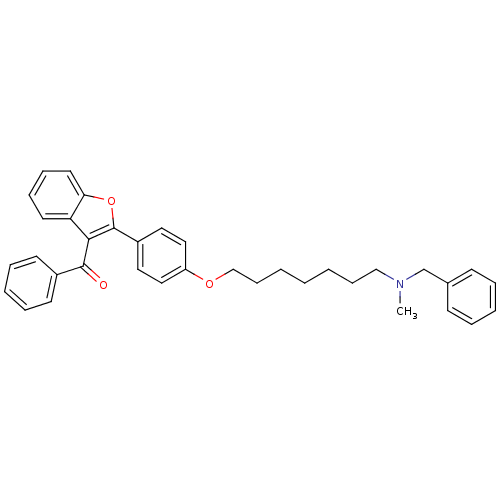 ((2-{4-[7-(benzylmethylamino)heptyloxy]phenyl}benzo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394567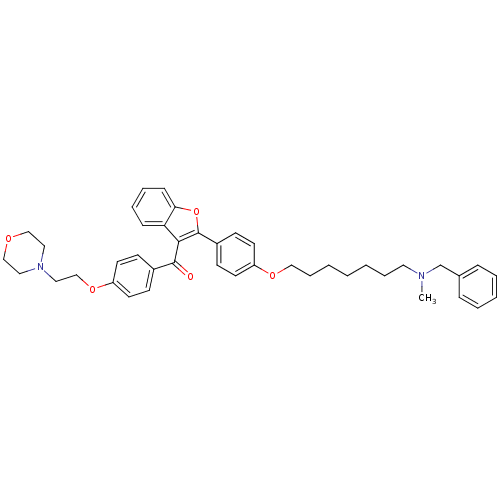 (CHEMBL2160225) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394567 (CHEMBL2160225) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394568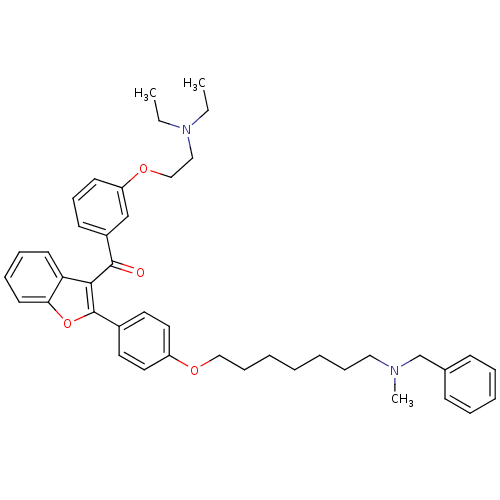 (CHEMBL2160223) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394569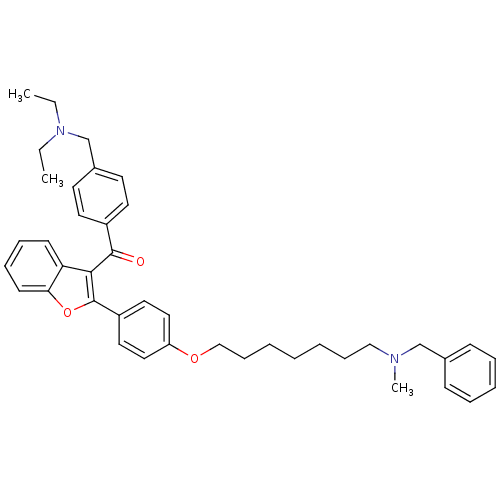 (CHEMBL2160222) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394570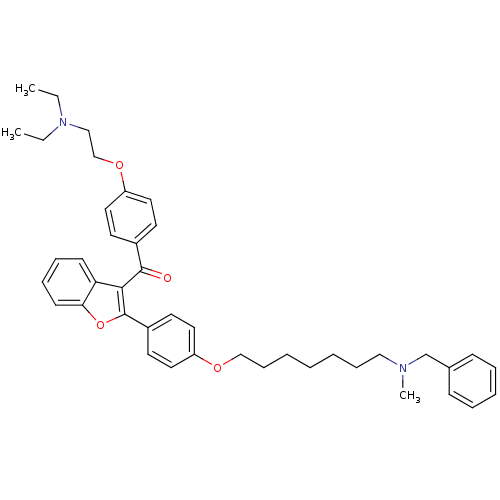 (CHEMBL2160224) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394572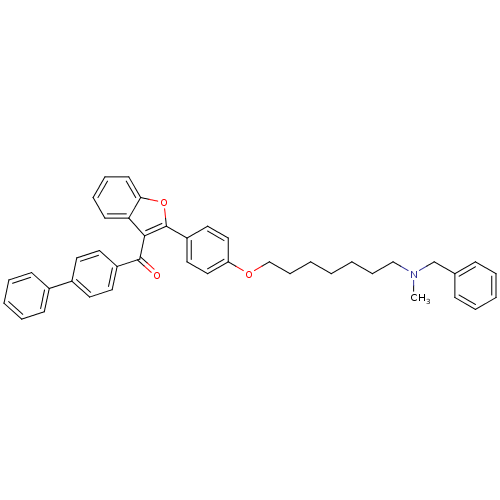 (CHEMBL2160219) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394568 (CHEMBL2160223) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50261202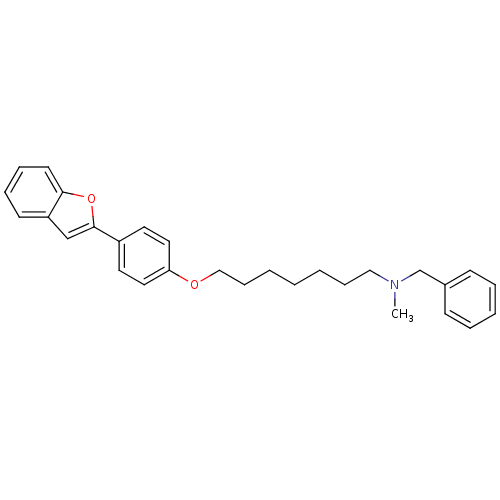 (CHEMBL497755 | [7-(4-Benzofuran-2-yl-phenoxy)hepht...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394572 (CHEMBL2160219) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394582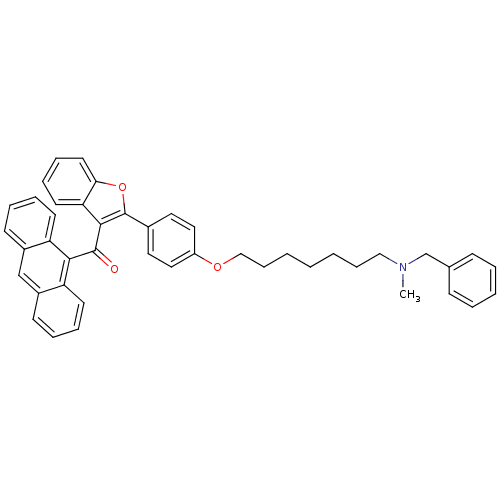 (CHEMBL2160220) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394581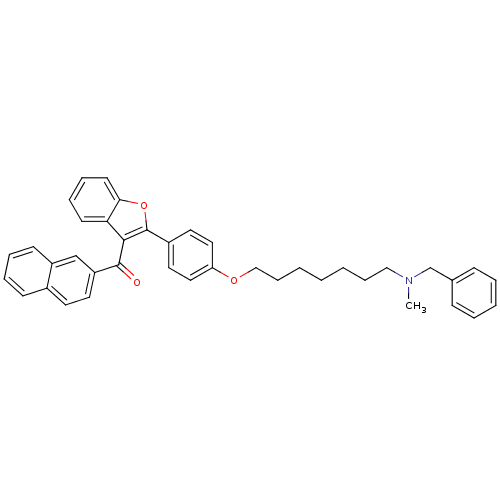 (CHEMBL2160218) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394580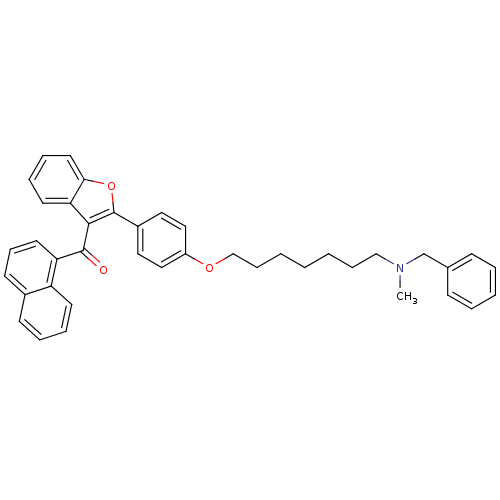 (CHEMBL2160217) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50261202 (CHEMBL497755 | [7-(4-Benzofuran-2-yl-phenoxy)hepht...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50261233 ((2-{4-[7-(benzylmethylamino)heptyloxy]phenyl}benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394577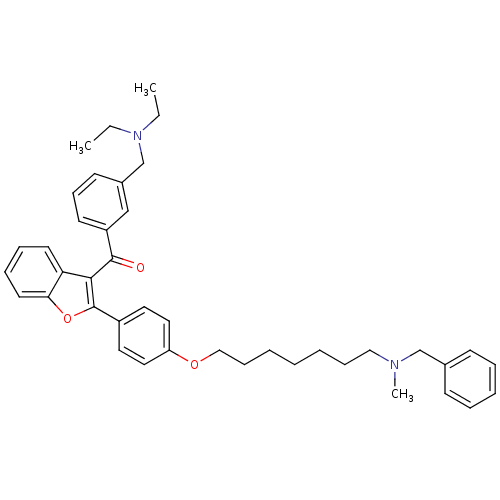 (CHEMBL2160221) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394569 (CHEMBL2160222) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394577 (CHEMBL2160221) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394582 (CHEMBL2160220) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394581 (CHEMBL2160218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394580 (CHEMBL2160217) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394570 (CHEMBL2160224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50467895 (CHEMBL4280262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50467897 (CHEMBL4287482) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM50467895 (CHEMBL4280262) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50467892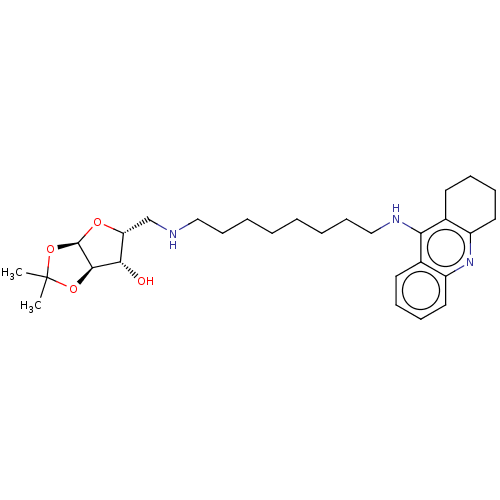 (CHEMBL4283717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50467898 (CHEMBL4282214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50467896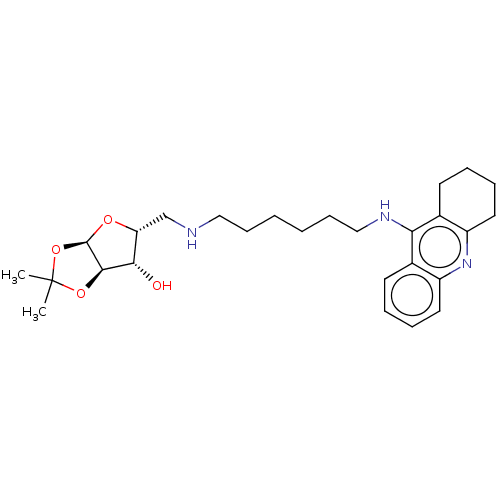 (CHEMBL4287068) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50467894 (CHEMBL4294287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM50467891 (CHEMBL4279148) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM50467892 (CHEMBL4283717) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM50467898 (CHEMBL4282214) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM50467896 (CHEMBL4287068) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM50467894 (CHEMBL4294287) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM50467897 (CHEMBL4287482) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM50467890 (CHEMBL4290902) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394563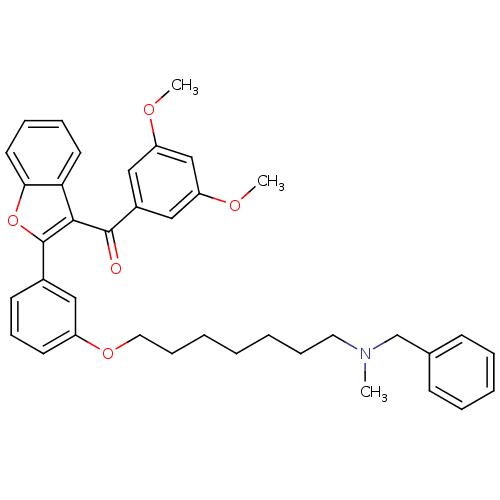 (CHEMBL2160234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM50467893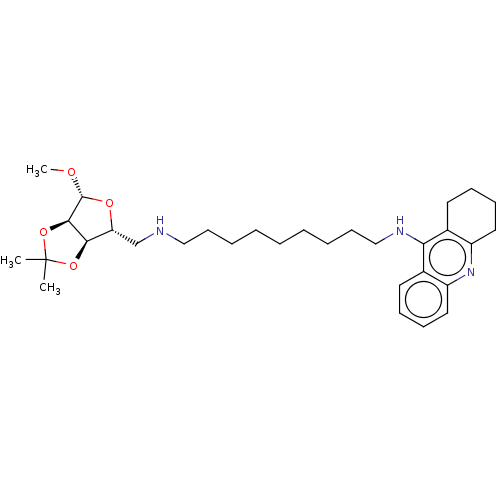 (CHEMBL4276832) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in Swiss Webster mouse whole blood serum using butyrylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50467893 (CHEMBL4276832) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50467891 (CHEMBL4279148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50467890 (CHEMBL4290902) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... | Bioorg Med Chem 26: 5566-5577 (2018) Article DOI: 10.1016/j.bmc.2018.10.003 BindingDB Entry DOI: 10.7270/Q2B56NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394564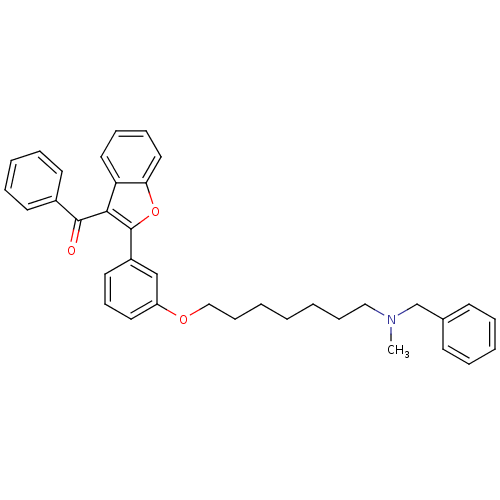 (CHEMBL2160227) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's met... | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394565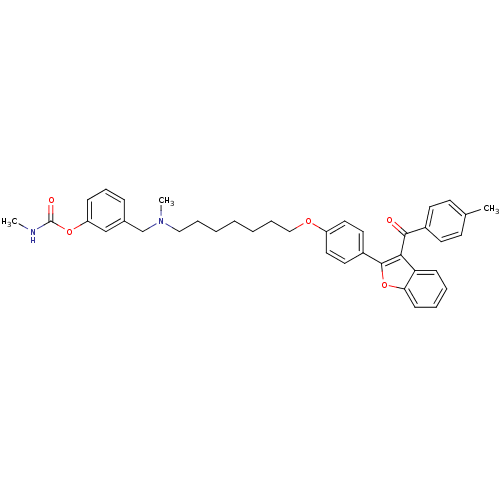 (CHEMBL2160226) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's met... | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394566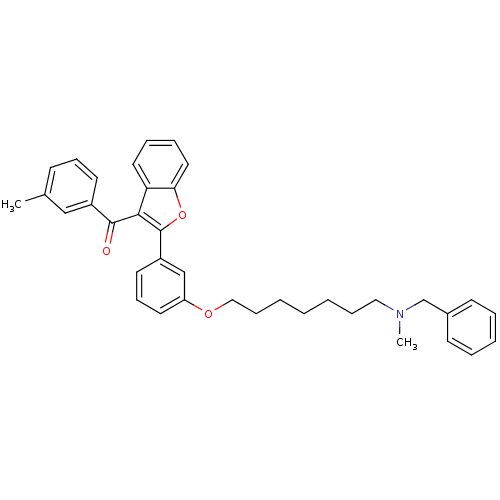 (CHEMBL2160228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394565 (CHEMBL2160226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394571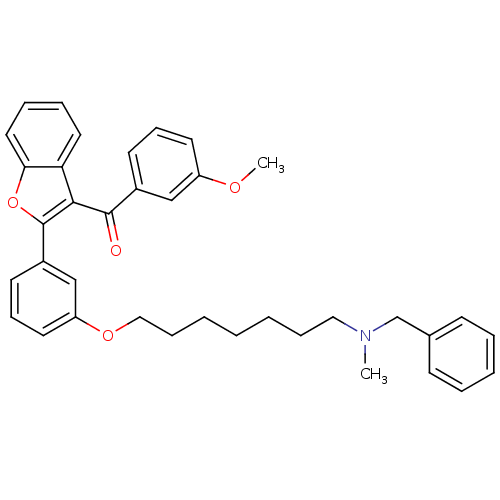 (CHEMBL2160231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |