

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268472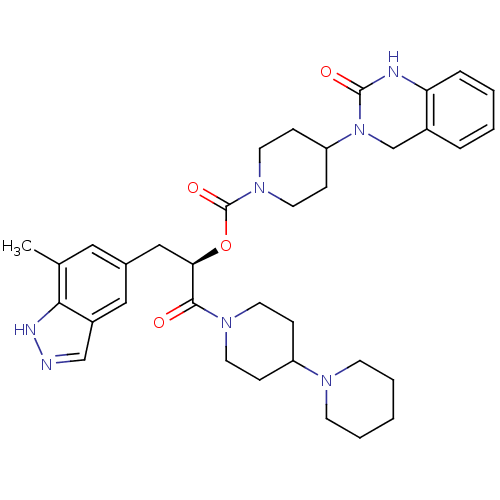 ((R)-1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-inda...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268471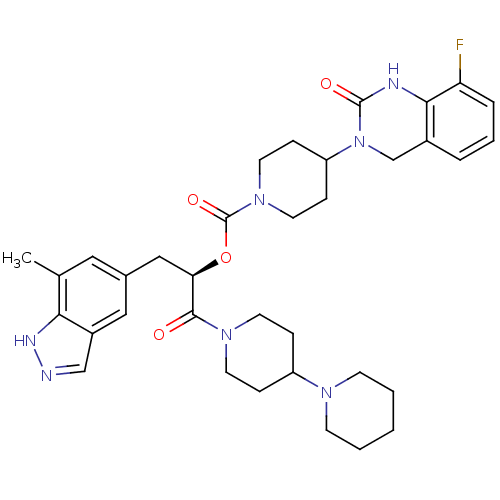 ((R)-1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-inda...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268474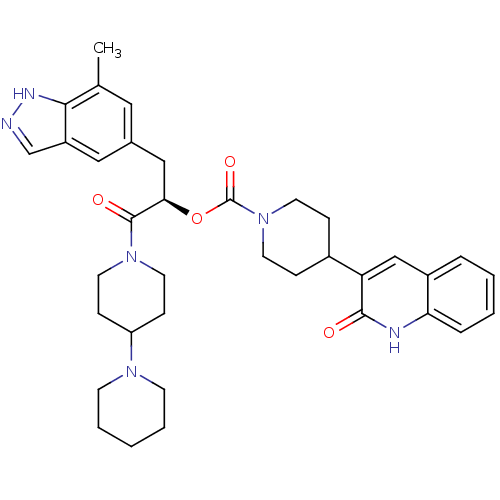 ((R)-1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-inda...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268481 ((R)-1-(4-cyclohexylpiperazin-1-yl)-3-(7-methyl-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268473 ((R)-1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-inda...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268480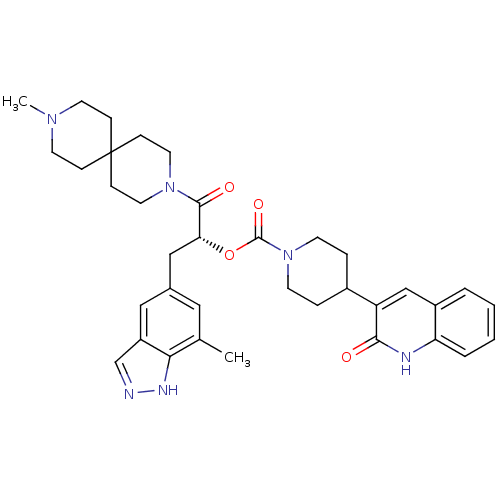 ((2R)-3-(7-methyl-1H-indazol-5-yl)-1-(9-methyl-3,9-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268479 ((R)-1-(4-(5,6-dihydropyridin-1(2H)-yl)piperidin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268475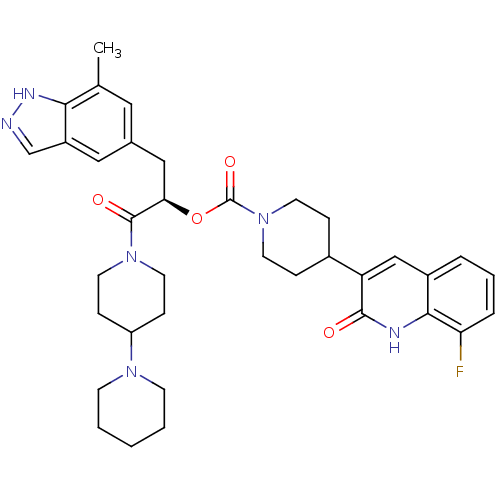 ((R)-1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-inda...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268482 ((R)-3-(7-methyl-1H-indazol-5-yl)-1-oxo-1-(4-(pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268477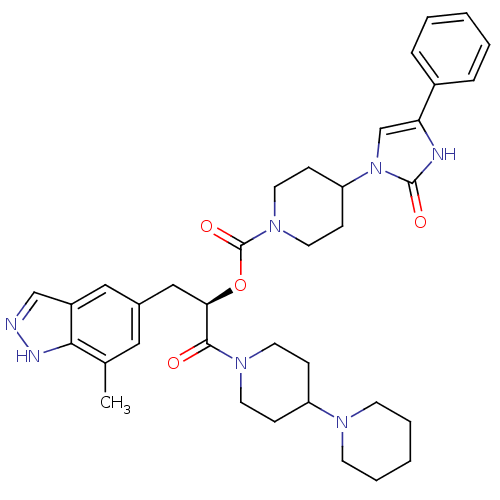 ((R)-1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-inda...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268483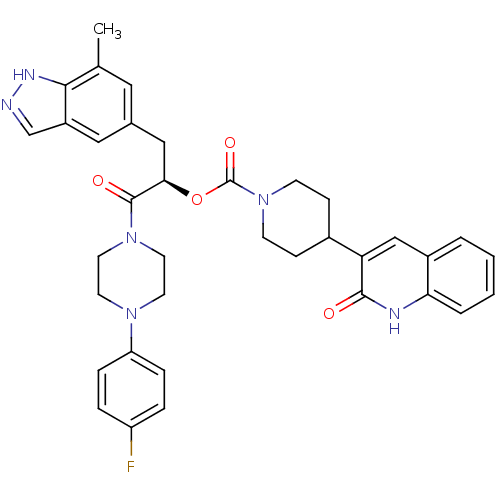 ((R)-1-(4-(4-fluorophenyl)piperazin-1-yl)-3-(7-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268476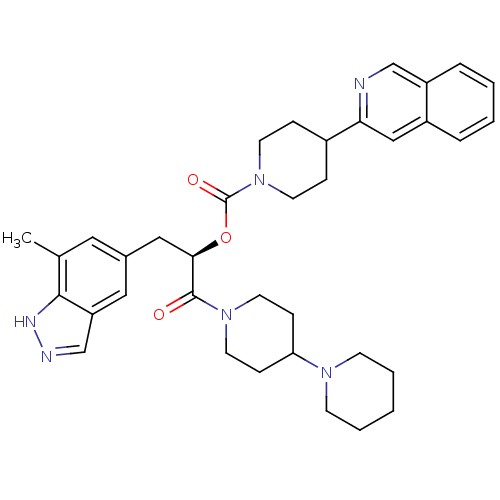 ((R)-1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-inda...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268478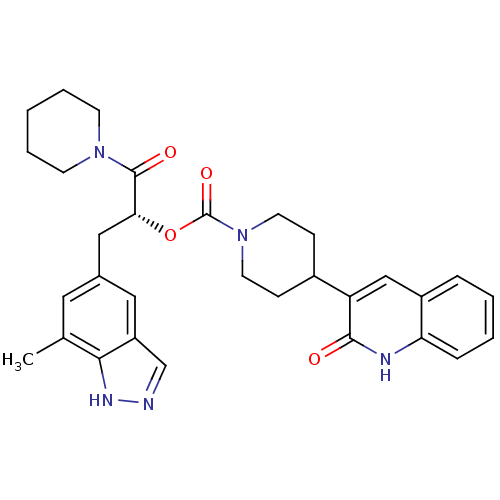 ((R)-3-(7-methyl-1H-indazol-5-yl)-1-oxo-1-(piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned CGRP receptor expressed in mouse E10 cells assessed as inhibition of CGRP-induced cAMP production | Bioorg Med Chem Lett 19: 3555-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.150 BindingDB Entry DOI: 10.7270/Q2WM1DBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171275 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50241125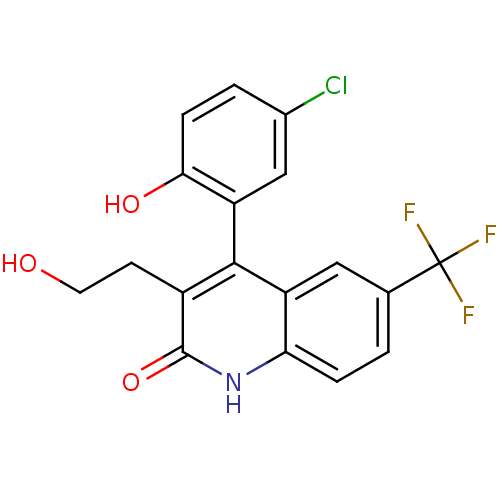 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 prepared from baculovirus-infected insect cells using 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171277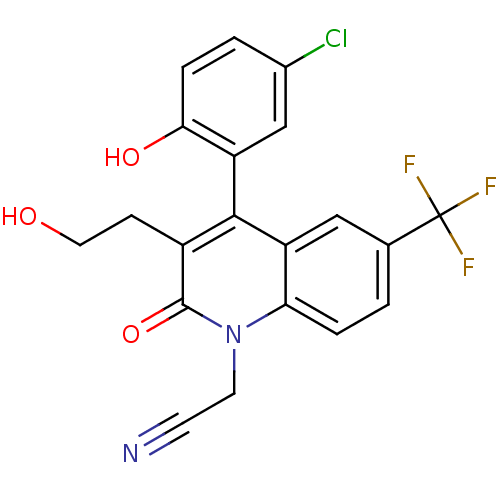 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 prepared from baculovirus-infected insect cells using 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C19 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171284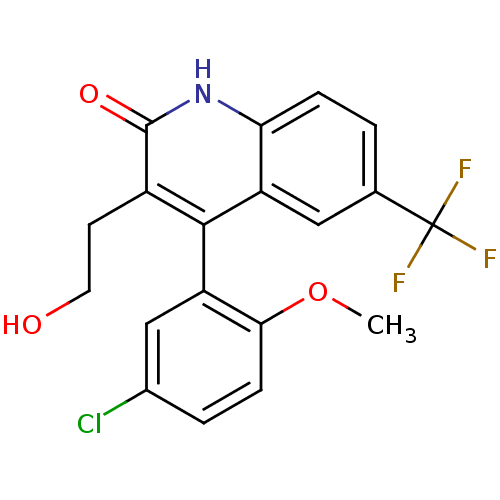 (4-(5-Chloro-2-methoxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using 7-benzyloxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50171282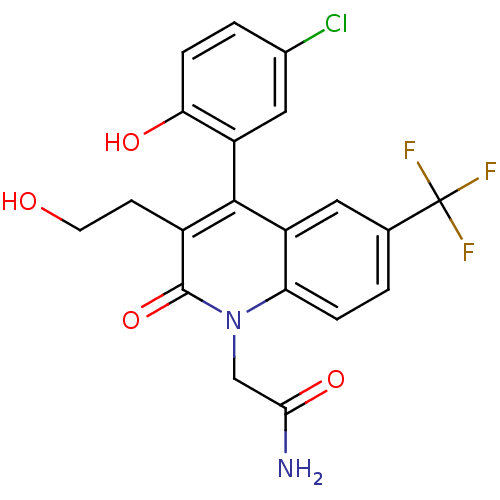 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C19 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50171280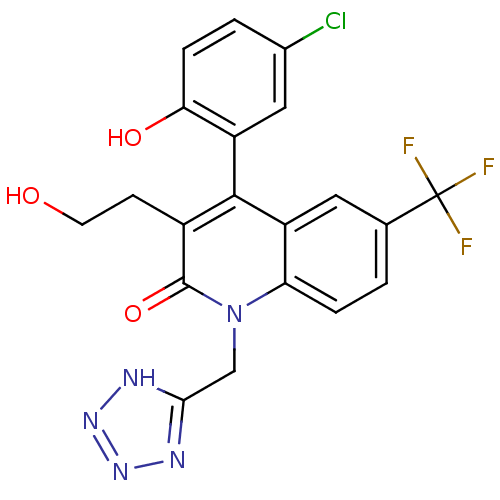 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C19 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171278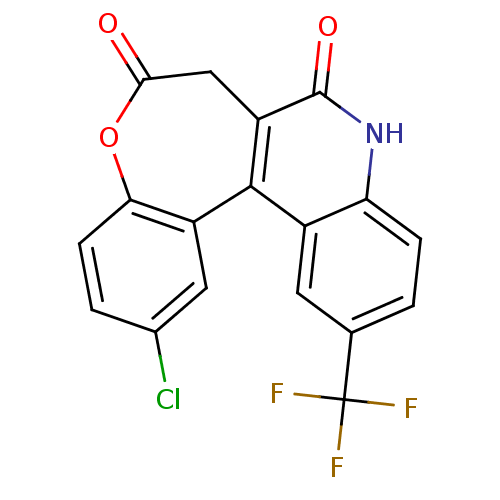 (12-Chloro-2-trifluoromethyl-5,7-dihydro-9-oxa-5-az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using resorufin benzyl ether | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using 7-benzyloxy- 4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 prepared from baculovirus-infected insect cells using 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using resorufin benzyl ether | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C9 prepared from baculovirus-infected insect cells using 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171283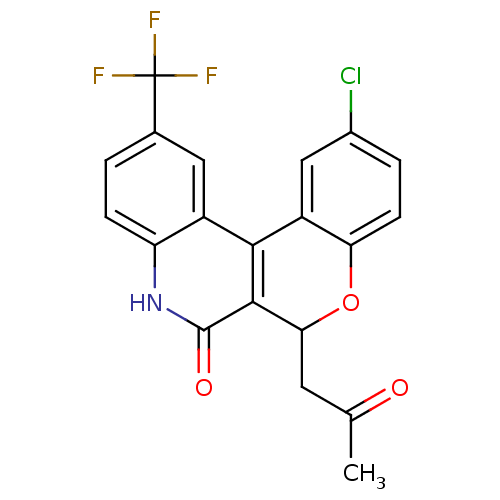 (2-Chloro-6-(2-oxo-propyl)-11-trifluoromethyl-6,8-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2D6 prepared from baculovirus-infected insect cells using 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7- methoxy-4-meth... | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2C19 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using 7-benzyloxy- 4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 1A2 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using 7-benzyloxy- 4-trifluoromethylcoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2D6 prepared from baculovirus-infected insect cells using 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methy... | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171279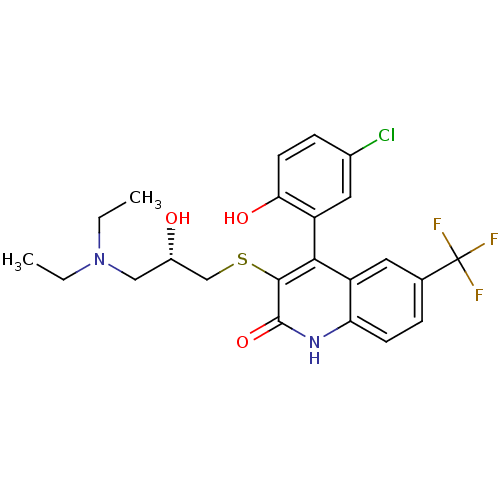 (4-(5-Chloro-2-hydroxy-phenyl)-3-[3-((S)-diethyl-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50171277 (CHEMBL195561 | [4-(5-Chloro-2-hydroxy-phenyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 1A2 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using resorufin benzyl ether | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171276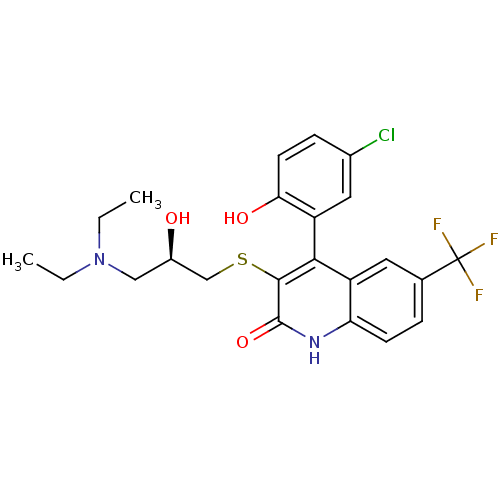 (4-(5-Chloro-2-hydroxy-phenyl)-3-[3-((R)-diethyl-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50171281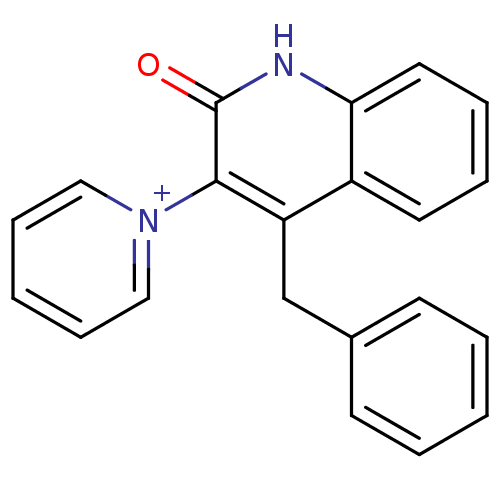 (1-(4-Benzyl-2-oxo-1,2-dihydro-quinolin-3-yl)-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cytochrome P450 2C9 | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50241125 ((+)-4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 3A4 prepared from baculovirus-infected insect cells using resorufin benzylether | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 1A2 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2D6 prepared from baculovirus-infected insect cells using 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7- methoxy-4-meth... | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50171282 (2-[4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 2D6 prepared from baculovirus-infected insect cells using 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7- methoxy-4-meth... | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50171280 (4-(5-Chloro-2-hydroxy-phenyl)-3-(2-hydroxy-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition against Cytochrome P450 1A2 prepared from baculovirus-infected insect cells using 3-cyano-7-ethoxycoumarin | Bioorg Med Chem Lett 15: 4286-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.056 BindingDB Entry DOI: 10.7270/Q2K64HMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||